2023 Executive War College Agenda
Here is the 2023 Agenda, with announced session Titles and Speakers for the EWC Conference.
After you register, this data will also be available on your Smart phone in the EWC Event App (Whova.)
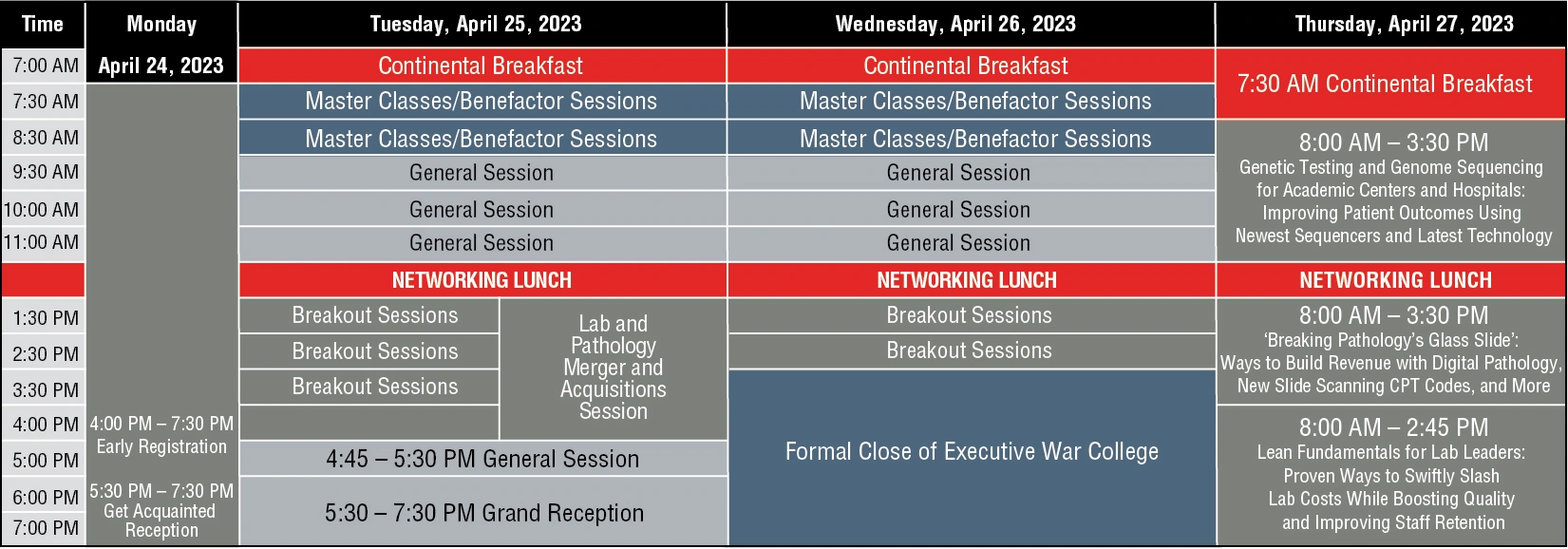
Monday, April 29 (Top)
REGISTRATION
4:00 PM - 7:30 PM
Early Registration
RECEPTION
5:30 PM - 7:30 PM
Get Acquainted Reception sponsored by CORE Academy Dx
Tuesday, April 30 (Top)
REGISTRATION
7:00 AM - 5:30 PM
Registration
BREAKFAST
7:00 AM - 9:30 AM
Continental Breakfast sponsored by ADSC
BENEFACTOR SESSIONS
7:30 AM - 8:20 AM
Thermo Fisher Scientific, Wave HDC, Quadax, Coronis, SYNERGEN Health
BENEFACTOR SESSION
7:30 AM - 8:20 AM
Thermo Fisher Scientific
Evidence and Application of Pharmacogenetic Testing to Drive Precision Medicine
Precision medicine holds the promise of leaving behind the one-size fits all approach to pharmacotherapy for more patient personalized treatment plans that take into account lifestyle, behaviors, polypharmacy and genetic profiles. An important part of this medication personalization is identifying pharmacogenes that can influence drug metabolism or side effect risk. This session will introduce the agencies and scientific consortiums that support pharmacogenetics (PGx) testing and the clinical trials and economic evidence surrounding PGx. Further, this session will highlight how Genomind has deployed PGx testing, decision support software, and clinical education for mental health.

Celeste Stone
Senior Manager, Business Development,
Thermo Fisher Scientific,
Lexington, KY

Russell Amato
PhD, Senior Medical Science Liaison,
Genomind,
King of Prussia, PA
BENEFACTOR SESSION
7:30 AM - 8:20 AM
Quadax, Inc.
Maximize Insurance Reimbursement Using Actionable Analytics
BENEFACTOR SESSION
7:30 AM - 8:20 AM
Coronis Health
Converting Data into Cash
Everyone has BILLING DATA. It’s about what you do with it to generate more cash.
This session will discuss the opportunities underpinning data and reports to produce usable information in the generation of criteria for action to collect more cash — plus beyond RCM to more broadly manage labs’ lines of business and making the tough decisions about what products and services to deliver in the laboratory or pathology marketplace. Everything from basic reporting’s value to the intense yields produced by putting Artificial Intelligence to work.
BENEFACTOR SESSION
7:30 AM - 8:20 AM
SYNERGEN Health
Fuel Lab Growth with Digital Revenue Cycle Solution
SYNERGEN Health, an industry-leading provider of technology-enabled revenue cycle services and solutions for healthcare organizations, and PreciseMDX, a leader in transformative digital health experiences, have formed a strategic partnership to provide laboratories with a seamless, end-to-end revenue cycle solution to bolster operational efficiencies and scalability, and fuel growth.
In this session, attendees will learn how this new digital revenue cycle solution combats current RCM challenges for laboratories and diagnostics. Using enhanced business intelligence, automation and advanced analytics, this new end-to-end solution transforms revenue cycle while improving efficiency and connectivity, and providing visibility into financial performance.

Sunil Konda
Vice President, Product
SYNERGEN Health,
Dallas, TX

Mark Dorner
Co-Founder & CEO
PreciseMDX
Los Angeles, CA
MASTER CLASS
7:30 AM - 8:20 AM
Leveraging Real Time Operational Lab Data Across 20 Hospitals to Track Workflow, Staff Productivity, and Manage How Physician Clients Benefit from Lab Testing
The presenters will share how MultiCare is leveraging hc1 Insights and other technology to drive accountability, workflow changes and education paths in the health system’s lab to be one of the Most Wired Health Care Organizations.
In this presentation, you will learn how MultiCare selected a tool that could take information from disparate systems into one, made it actionable and ensured it was being used by the right people for key decision-making processes.

Zac Zahara
COO System Lab,
MultiCare Health System

Jennifer Maxwell
Executive Director of Client Success, hc1, Indianapolis, IN
MASTER CLASS
7:30 AM - 8:20 AM
The importance of Pre-Analytical and Post-Analytical service functions in the Clinical & Anatomic Laboratory marketplace: Over 50%-60% of the Costs – and – 80%-90% of the Customer Service and Management Challenges
The objective of this session is to allow the participants to: A) be aware of the competitively necessary functions & services included in these Pre-Analytical and Post-Analytical activities, and B) develop management disciplines to allow you and your management teams to Cost Efficiently monitor and report the most important KPI’s and trends while taking action on areas that need to be addressed in a Continuous Improvement manner.
MASTER CLASS
7:30 AM - 8:20 AM
Five Issues that Keep New CLIA Laboratory Directors Up at Night: Compliance Requirements, Changes in Enforcement, Overlooked Sources of Risk

Lindsay Strotman
PhD,
Laboratory Director,
Lighthouse Laboratory Services,
Louisville, KY

Nora Hess
MBA, MT (ASCP), PMP,
Senior Consultant, Accumen
BENEFACTOR SESSIONS
8:30 AM - 9:20 AM
Change Healthcare, U.S. HealthTek, Telcor
BENEFACTOR SESSION
8:30 AM - 9:20 AM
Change Healthcare
The Data is In: Emerging Trends and Best Practices for Laboratory Testing

Caroline Juarez
Product Director,
Change Healthcare,
Denver, CO.
BENEFACTOR SESSION
8:30 AM - 9:20 AM
U.S. HealthTek
Leveraging IT Investments: Maximize Your Lab’s Value and Performance
Learn how strategic investments into IT can directly impact your company’s value and increase performance, with proper planning. We’ll cover scenarios ranging from Mergers & Acquisitions to standard best practices, with a Q&A to address specific audience questions.
BENEFACTOR SESSION
8:30 AM - 9:20 AM
TELCOR
Lessons Learned One Year Later: Strategies and Solutions for Managing the Complex Billing Requirements of Prior Authorization and Appeals While Minimizing Cost
Two of the most complex billing scenarios are managing prior authorizations and claim appeals. Both can require significant intervention, tracking, and follow-up. Payer and provider requirements are not standard, complicating the entire process. By working with customers across the country, TELCOR truly understands the nuances of obtaining a prior authorization and making the appeals process as streamlined as possible. During this session, TELCOR will share how to:
- Create a prior authorization process that is as automated as possible while balancing the need for manual intervention.
- Design visibility around prior authorization requests and appeals submissions to ensure everything is tracked and follow-up is timely and efficient.
- Manage, streamline, and automate the entire appeals process ensuring your lab is collecting as much as possible.
MASTER CLASS
8:30 AM - 9:20 AM
Today’s Supply-Demand Gap for Skilled Pathologists: How it Happened, Why It Will Continue, and How Innovative Labs Attract, Hire, and Retain Top Talent

Dr. Stanley Robboy
MD, FCAP, FFPath FRCPI (Hon), FRCPath (Hon),
Professor Emeritus of Pathology,
Duke University Medical Center
MASTER CLASS
8:30 AM - 9:20 AM
Automating the Patient Experience at Time of Genetic Test Order: Effective Approaches to Engage Genetic Counselors and Benefits Investigation to Speed Patient Acceptance
Diagnostics centered around genetic, genomic, and molecular testing can be a complex business with many unique and not-so-unique challenges. Maximizing automation is a critical means for labs that offer this type of testing to keep up with ever-changing payor policies and requirements and has become table stakes to maintain a competitive edge. This includes a blend of well-executed patient and physician engagement programs, as well as market access knowledge that all directly ties into the need for an intelligent revenue cycle management system to manage all these critical dependencies. In this session, Mr. Ross and Ms. Agostinelli will explore winning physician engagement strategies and techniques designed to increase ordering volumes, maximize clean claims, and automate denials and appeals management to maximize reimbursement. They will discuss novel ways to engage genetic counselors to better anticipate and respond to payor behavior and will report on one such program that leverages genetic counselor knowledge to have greater confidence and predictability of reimbursement success at a payor/plan and test level. They will also discuss the most effective patient communication touchpoints and technology enablers to assist patients in preparing for out-of-pocket expenses and increase test completion rates.
Our speakers will also examine other opportunities for intelligent automation and process enhancement affecting the patient experience, including prior authorizations, insurance discovery, benefits determination, and successful appeal strategy concepts.

Harley Ross
Chief Commercial Officer,
XiFin, Inc.
San Diego, CA

Heather Agostinelli
Vice President,
Strategic Revenue Operations,
XiFin, Inc.
San Diego, CA
MASTER CLASS
8:30 AM - 9:20 AM
Progressive Steps to Solve Pain Points in Lab Revenue Cycle Management: Deploying AI-Powered Solutions, Improving Cash Flow Projections, and Collecting Accurate, Complete Data with the Lab Request

Ted Knauf
Sr. Director of Business Operations,
Gravity Diagnostics,
Covington, KY

Michael Tarwater
Vice President of
Information Technology,
Gravity Diagnostics,
Covington, KY
MASTER CLASS
8:30 AM - 9:20 AM
Delivering Added Value Pathology Services to Oncologists: Bringing Sophisticated Cancer Testing In-house to Improve TAT, Patient Care, and Grow Revenue
MASTER CLASS
8:30 AM - 9:20 AM
Achieving Faster Sepsis Diagnosis in the Emergency Department: Early Experience with the Monocyte Distribution Width (MDW) Marker and Acceptance by ED and ID Physicians

Robert Patterson
MD, MPH
Medical Director of Pathology,
Lab Medicine & Lab Outreach,
Butler Health System,
Butler, PA
GENERAL SESSION
9:30 AM - 10:10 AM
Positioning Your Lab to Prosper by Serving Healthcare’s New Consumers, New Care Models, New Payment Models, and More!
GENERAL SESSION
10:10 AM - 10:40 AM
How Old School Lab Rules Evolved into New School Lab Rules and Ways to Transition Your Lab Through Today’s Disrupters in Healthcare and the Clinical Laboratory Marketplace
The lab is facing a world of disruptors the old school of best practices is requiring a new school of thought and innovation.
AM BREAK
10:40 AM - 11:00 AM
AM Break sponsored by Sysmex
GENERAL SESSION
11:00 AM - 11:35 AM
Is It Time for Retail Pharmacies and Clinical Labs to Start a Dating Relationship to Offer Value to Each Other?
As the healthcare landscape evolves, laboratories are seeking new ways to increase revenue, maximize reimbursement, and provide a seamless patient experience. One promising avenue for achieving these goals is through partnerships with pharmacies and specialty physician groups.
In this speaker session, our expert will share valuable insights on this growing trend, emphasizing the value of collaborating with pharmacies to achieve these goals. The speaker will discuss the recommended practices for laboratories to partner with pharmacies using collaborative practice agreements, which allow pharmacists to perform clinical tests and prescribe medications based on laboratory results.
The speaker will also cover current trends related to the gains in pharmacist prescriptive authority, which have expanded the scope of services that pharmacies can offer. This expansion has led to increased opportunities for laboratories to partner with pharmacies and specialty physician groups to provide innovative, patient-centered care.
Additionally, the session will review the important role of synchronized data, including electronic health records, test results, and claims data, for optimizing the patient experience in a point-of-care pharmacy clinical testing setting. By integrating these data sources, laboratories and pharmacies can work together to ensure that patients receive accurate diagnoses and effective treatments in a timely manner.
Attendees will leave this session with a deeper understanding of the benefits of laboratory-pharmacy partnerships, as well as practical strategies for establishing and maintaining successful collaborations. Whether you’re a laboratory professional, a pharmacist, or a healthcare provider looking to improve patient care and increase revenue, this session is a must-attend.
GENERAL SESSION
11:35 AM - 12:10 PM
Generating Value by Identifying Risk Signals in Longitudinal Lab Data: Opportunities in Big Data with Payers, Physicians, Pharma, and Bioresearch
hc1 Chairman and CEO Brad Bostic will discuss the current state of Big Data and the lab’s opportunity to participate. This presentation will address important trends in real-world data, how AI can add value, and the role labs can play in helping physicians, payers, and life sciences companies to gain an advantage. He will also share how emerging large language models, like ChatGPT, can transform healthcare delivery, and the potential for perilous consequences if AI models are left to run unchecked.
LUNCH
12:10 PM - 1:45 PM
Networking Lunch sponsored by ELLKAY
WORKSHOP
1:30 PM - 4:35 PM
Laboratory and Pathology Mergers & Acquisitions Workshop
Session Chair: Richard Cooper
What’s Changing with Buyers of Hospital Labs, Independent Labs, Genetic labs, and Pathology Groups
Panel Chair/Moderator: Richard Cooper
Panel: John Runk, Anil Asnani, Matthew Urbanek
Bringing Your Hospital Lab or Independent Lab to Market for the Optimal Price: Timing, Pitfalls, Showing the Best “Face”
Panel Chair/Moderator: Christal Contini
Panel: Christopher Jahnle, Gary Huff, Jeff Downs, Ven Aduana
Best Paths to Attracting Capital and Crafting Effective Growth Strategies that Support a High-Multiple Sales Valuation for Genetic Testing Labs
Panel Chair/Moderator: Melissa Butterworth
Panel: Bryan Firestone, Emily Johnson, Myles Standish
Understanding Regulatory Compliance and Operational Landmines That Often Disrupt Lab Sales and Closings
Panel Chair/Moderator: David Gee
Panel: Elizabeth Sullivan, Diana Voorhees, Chris Jahnle

Richard Cooper
JD,
CEO, GIG Consulting LLC,
Advanced Strategic Partners,
Chagrin Falls, OH

Christal Contini
JD,
Practice Chair,
McDonald Hopkins,
Cleveland, OH

Bryan Firestone
Founder, U.S. HealthTek,
Haymarket, VA

Melissa Butterworth
President & CEO,
Advanced Strategic Partners,
Hollywood, FL

Elizabeth Sullivan
Attorney, McDonald Hopkins,
Cleveland, OH

Christopher Jahnle
Managing Director,
Haverford Healthcare Advisors,
Radnor, PA

Anil Asnani
SVP Strategy & Corporate Development, LabCorp
Burlington, NC

John Runk
Director of Finance & Head of Corporate Development, PathAI,
Barrington, RI

Matthew Urbanek
Senior Vice President, Finance,
Eurofins Clinical Diagnostics,
Lenexa, Kansas

Gary Huff
President/Owner, Take Charge LLC,
Hampstead, NC

Myles Standish
Consultant,
Sound Medical Laboratory/Pacific Physician’s Laboratory Lab,
Redmond, WA

Emily Johnson
Emily Johnson, JD,
Member, McDonald Hopkins LLC,
Chicago, IL

Jeff Downs
Vice President,
Strategic Relationships, Accumen,
Scottsdale, AZ

Diana Voorhees
MA, MLS, SH, CLCP, CPCO,
Principal/CEO
DV & Associates, Inc.
Salt Lake City, UT

Ven Aduana
MD,
Chairman, Chief Medical Officer, Versant Diagnostics,
Chicago, IL
ROUNDTABLE
1:30 PM - 3:20 PM
Lab Informatics and Lab CIO Roundtable

Josh Kramer
Managing Partner,
Leap Consulting Group,
Teaneck, NJ

Merve Ozkus
SVP, Chief Information Officer,
BioReference Health,
Woodland Park, NJ
PANEL
1:45 PM - 2:35 PM
Legal Panel: Important Developments in Laboratory Legal, Regulatory, and Compliance Requirements

Jeffery Sherrin
Of Counsel,
O’Connell & Aronowitz, P.C.

Paul Garcia
Esq., Partner,
Hooper Lundy & Bookman,
Los Angeles, CA
BREAKOUT SESSION
1:45 PM - 2:35 PM
Using the Lab-Initiated Care Model’ to Achieve Earlier, More Proactive Diagnoses of Chronic Kidney Disease in Patients with Co-Morbidities of Diabetes and Heart Failure, Quantifiable Role of Clinical Labs as We Transition From ‘Business of Volume’ to ‘Business of Value’- Value Based Care

Khosrow Shotorbani
President, Executive Director,
Project Santa Fe Foundation- Lab 2.0, Salt Lake City, UT

James Crawford
MD, PhD,
Senior Vice President of Laboratory Services, Northwell Health, Greenvale, NY
BREAKOUT SESSION
1:45 PM - 2:35 PM
How Automated Tools Increase Collected Revenue, Produce More Claims, and Require Less Labor: Unleashing the Power of AI and Machine Learning in Coding/Billing/Collections

Guillermo Martinez-Torres
MD,
President & Chief Physician Executive, NorDx,
Scarborough, Maine

Dean Paluch
CRO,
Wave HDC,
Butler, NJ
BREAKOUT SESSION
1:45 PM - 2:35 PM
Useful Insights from the Design, Construction, and Operation of a New High-Volume Laboratory: Managing Costs, Sustaining Quality, and Improving Patient Care
In this session, Dr. Genzen will be discussing a multi-year construction initiative which expanded ARUP’s laboratory footprint by over 200,000 sq feet. Inspired by Lean principles and ARUP’s commitment to continuous quality improvement, this project engaged laboratory operations, facilities, quality, and engineering teams to optimize processes in support of increasing efficiency and reducing turn-around-time. Projects included pre-analytic, analytic, and post-analytic automation, as well as re-thinking traditional workflows to streamline and improve the specimen journey both within and across laboratory sections. Particular focus was placed on green building principles and maximizing recycling across operations. Throughout the presentation, Dr. Genzen will share metrics on the overall impact of the project on laboratory turn-around-time and continuous quality improvement initiatives. Please join us for this highly informative and engaging business case study!

Jonathan Genzen
MD, PhD,
Chief Medical Officer / Professor,
ARUP Laboratories / Univ. of Utah,
Salt Lake City, UT
BREAKOUT SESSION
1:45 PM - 2:35 PM
New Opportunities for Local Labs and Hospitals to Scale Genome Sequencing Modalities to Utilize Capacity, Advance Patient Care, and Open Doors to More Reimbursement
BREAKOUT SESSION
1:45 PM - 2:35 PM
Lab Staff Recruiting, Hiring, and Retention in Today’s Competitive Market: How We Differentiate Our Lab, Attract Qualified Candidates, and Build Loyalty and Commitment
BREAKOUT SESSION
2:45 PM - 3:35 PM
Early Successes and Lessons Learned in Launching a National Digital Pathology Network in the UK
In this session, Dr Williams will discuss an ambitious and innovative digital pathology programme launched in the United Kingdom, and how a single site, single hospital digital pathology deployment has grown into flourishing regional and national clinical networks.

Bethany Williams
Lead for Training, Education and Public/Patient Involvement,
National Pathology Imaging Co-Operative
BREAKOUT SESSION
2:45 PM - 3:35 PM
ISO15189 Accreditation – Your Key to Opening Expanded Global Testing Markets
ISO15189 has become the accepted global standard and accreditation recognizes the acceptability of testing performed. As an example, Governments relied on it for acceptance of Covid testing results. Why your lab should consider being accredited, a case study discussing the benefits of accreditation from Alpha Laboratories. Also included is a review of the changes in the 2022 edition of ISO15189.

Sheila Woodcock
MBA, ART, FCSMLS(D),
President & Principal Consultant,
QSE Consulting Inc.,
Nova Scotia, Canada

Andrea Park
MLT,
Alpha Labs,
Ontario, Canada
PANEL
2:45 PM - 3:35 PM
Panel: State of the IVD Industry: What’s Working Best in Today’s Post-Pandemic Market for Lab Vendors and Their Customers

Bob McGonnagle
Publisher, CAP Today,
College of American Pathologists,
Northfield, IL

Bruce Carlson
Senior Vice President,
Kalorama Information,
Arlington, VA

Larry Worden
Principal,
IVD Logix LLC,
Dallas, TX

Jim Kathrein
Sr. Commercial Business Advisor/Consultant,
Kathrein Consulting,
Helena, MT
BREAKOUT SESSION
2:45 PM - 3:35 PM
CDC Update: Recent CLIAC Activities, Public/Private Laboratory Collaborations, and Resources
BREAKOUT SESSION
2:45 PM - 3:35 PM
Point-of-Care Testing (POCT) In Six Hospitals Plus Five Free-Standing EDs: Using Patient-Centric Services to Reduce Unnecessary Tests, Improve Patient Care, and Capture All Test Results
BREAKOUT SESSION
2:45 PM - 3:35 PM
Building Lab Outreach Market Share While Creating Everlasting Doctor Loyalty
Discussion will surround development of successful sales strategies based on laboratory platform and marketplace. The successful sales team understands laboratory operations, IT, the marketplace, company initiatives and the “business” of laboratory medicine. A successful sales team is a knowledgeable sales team.
Sales teams generally market less than 30% to 40% of the potential client base in any given market. That segment is commonly “recycled” by sales executives. Why is it that the vast majority of clients are left untouched and how do we access them?
Developing client loyalty is a laboratory-wide effort and effective strategies on this will be covered. How to break the cycle of losing business and create healthy, long-lasting client relationships and loyalty.
PANEL
3:45 PM - 4:35 PM
Everything You Need to Know about Washington, DC: Issues of Concern to Labs, Including PAMA, VALID Act, FDA, OIG, CMS, Congress, and More

John Kolozsvary
Chief Executive Officer,
Joint Venture Hospital Laboratories,
Allen Park, MI

Susan Van Meter
President, American Clinical Laboratory Association

Jeff Allen
PhD,
President and CEO,
Friends of Cancer Research

Emily Volk
MD, MBA, FCAP,
President,
College of American Pathologists,
Northfield, Ill

Laura Tafe
MD, Associate Professor of Pathology & Laboratory Medicine,
Dartmouth Hitchcock Medical Center,
Lebanon, NH
PANEL
3:45 PM - 4:35 PM
Using Patient Engagement and Data to Drive Lab Growth, New Revenue Opportunities, and Improved Outcomes
While patient engagement and data analytics have long been healthcare buzzwords, how can labs leverage them to drive growth? In this session, we will bring together industry leaders to discuss real examples of how the combination of patient engagement and data analytics can not only benefit patients, but also drive growth and generate new economic value for both health system and commercial laboratories.
We will share insider views on how this powerful combination is being used to develop new services and strategic initiatives. We will specifically touch on how this can be used to introduce population health programs, direct-to-consumer testing platforms, wellness campaigns, care programs, and more, as well as make the economic case for them within lab organizations.
Attendees will come away from the session armed with an understanding of how patient engagement and data analytics can align a lab’s interests in ultimately improving patient outcomes while producing new revenue opportunities and growth for the lab.

Shally Madan
COO & Co-Founder,
Luminate Health,
San Mateo, CA

Amy Nichols
VP, Customer Applications and Analytics,
PathGroup,
Windsor, CO
BREAKOUT SESSION
3:45 PM - 4:35 PM
Genetic Test Stewardship: A Collaborative Approach to Improve Test Coverage, Revenue, and Patient Care

Jessie Conta
Laboratory Stewardship Consultant,
PLUGS / Pickhandle Consulting,
Seattle, WA

Jane Dickerson
PhD, Division Head, Lab Medicine,
Seattle Children’s Hospital,
Seattle, WA
BREAKOUT SESSION
3:45 PM - 4:35 PM
Current State of Private Practice Pathology: Disruptive Trends and Action Steps to Protect Pathologist Income and Boost Practice Revenue
BREAKOUT SESSION
3:45 PM - 4:35 PM
Implementing Epic Beaker LIS in 40+ Facilities Across Three States: Lessons from Accomplishing the Incredible in Only Four Implementation Waves

Deanna Franke
PhD, DABCC,
Technical Director – Core Laboratory,
Atrium Health,
Gastonia, North Carolina

Jamel Giuma
President & CEO,
JTG Consulting Group,
Miami, FL
BREAKOUT SESSION
3:45 PM - 4:35 PM
Why Today’s Evolution of Multi-Hospital Health Systems Requires an Effective Lab Outreach Program for Health Systems’ Strategies to Succeed
BREAKOUT SESSION
3:45 PM - 4:35 PM
Repurposing the COVID-19 Workflow in a Post-COVID Infectious Disease Lab
GENERAL SESSION
4:45 PM - 5:30 PM
What’s Ahead for Clinical Laboratories and Anatomic Pathology Laboratories in the Age of Consumerism, Value-Added Reimbursement, and Next-Generation Sequencing

Robert Michel
President,
The Dark Intelligence Group,
Austin, TX

Bob McGonnagle
Publisher, CAP Today,
College of American Pathologists,
Northfield, IL

Stan Schofield
Managing Principal,
The Compass Group,
Scarborough, ME

Alfred Lui
MD,
President and Medical Director,
Innovative Pathology Medical Group,
Torrance, CA
Wednesday, April 26 (Top)
REGISTRATION
7:00 AM - 3:45 PM
Registration
BREAKFAST
7:00 AM - 9:30 AM
Continental Breakfast sponsored by Mayo
BENEFACTOR SESSIONS
7:30 AM - 8:20 AM
XiFin, AIMA, Clinisys
BENEFACTOR SESSION
7:30 AM - 8:20 AM
XiFin
Harnessing the Power of RCM Data and Insights to Transform Your Business and Operational Strategies
This panel discussion will focus on the role of RCM data and analytics in transforming laboratory business and operational strategies. The talk will cover how various stakeholders can use RCM data and analytics insights, including finance executives, revenue cycle leaders, commercialization efforts, and managed care professionals.
The panelists will discuss how finance executives can use analytics to make informed decisions about resource allocation, investment strategies, and overall financial management. They will also identify inefficiencies and cost-reduction opportunities. The moderator will explore the most common areas of revenue leakage with the panelists and provide examples of corrective actions that improve billing accuracy and optimize reimbursement rates.
The moderator will explore how RCM data and analytics can provide valuable insights into client health, allowing laboratory businesses to identify clients at risk of churn or low utilization. Panelists will describe how utilization data and payment patterns enable lab leaders to develop targeted retention strategies to improve client engagement and reduce churn.
Payor behavior is another critical area where RCM data and analytics can provide insights. Panelists will share examples of insights managed care professionals can utilize to negotiate better contracts, optimize reimbursement rates, and track utilization trends and coverage policies.
Accounts receivable valuations are another critical area that the panel will discuss and how laboratories can identify areas of inefficiency and develop strategies to improve cash flow and reduce write-offs.
In summary, the panel discussion will highlight how RCM data and analytics can be leveraged by various stakeholders in laboratory businesses. This data provides insights into key financial and operational metrics, which will inform strategic decision-making, drive higher profitability, optimize revenue streams, and capture market share.

Diana Richard
Sr. Director, Pathology and Strategic Development, XiFin,
San Diego, CA

Timothy Langford
Director of Billing,
Clinical Diagnostics US,
Eurofins Scientific

Heather Agostinelli
Vice President,
Strategic Revenue Operations,
XiFin, Inc.
San Diego, CA

Ben Conroy
Director of Molecular Revenue,
XiFin, Inc.
BENEFACTOR SESSION
7:30 AM - 8:20 AM
AIMA
Leveraging the Laboratory Revenue Cycle, a 2023 (and Beyond) Strategy Roundtable. Share, learn and collaborate Real talk about real examples and learning of medical necessity, MolDX, Genetic Billing Compliance, Automation in RCM and much more!
MASTER CLASS
7:30 AM - 8:20 AM
“New Class III CPT Codes for Digital Pathology: Why They Are a Trigger to Better Outcomes and Pathologist Reimbursement and How They Can Bring DP Patients to Your Lab”

Esther Abels
MSc, Biomedical Regulatory Health Science Expert,
Boston, MA
MASTER CLASS
7:30 AM - 8:20 AM
Competing with the National Lab Chains’ Patient Service Centers: A New National Network of Retail Clinics and Phlebotomy Options to Service Your Lab Customers
How do labs address their needs for specimen collections when the physician or healthcare provider does not draw in their office, lab does not have their own PSC network, or they sell direct to consumer?
Having an easy access draw site, convenient to where a patient can have their phlebotomy or specimen collections properly performed is an expensive proposition and even more difficult now due to staffing issues. Finding a solution that meets the compliance needs of the Anti-Kickback Statutes, while also affordable has been difficult if not impossible to manage if at all possible.
My One Medical Source (MOMS) is a solution to address this need and has origins from addressing this need from experience in building Cleveland HeartLab (now Quest). Addressing the need for greater access for labs to be able to direct patients with either a lab order or kit from their lab is how a fast start lab agreed to use MOMS as their national specimen collection solution.
2020 GeneSystems signed on with MOMS at the EWC 2022 to help address their needs for having a brick and mortar phlebotomy solution where they could help patients who ordered their advanced testing, to find a location near them to have their draw performed. Using the MOMS MAPs: Medical Access Point™ network, 2020 GeneSystems now has a national solution to help increase their ability to have specimens properly collected, prepared and shipped back to their lab for resulting.

Jonathan M. Cohen
President & CEO,
20/20 GeneSystems, Inc.,
Gaithersburg, MD

Bradley Seybert
President/Founder,
My One Medical Source (MOMS), Westlake, OH
MASTER CLASS
7:30 AM - 8:20 AM
Prostate Biopsy chip implementation – Improves Accuracy of Prostate Cancer Diagnoses, Reduces Lab Costs, and Shortens Pathologists’ Diagnosis time
BENEFACTOR SESSIONS
8:30 AM - 9:20 AM
Beckman Coulter Diagnostics, hc1, ELLKAY
BENEFACTOR SESSION
8:30 AM - 9:20 AM
Beckman Coulter Diagnostics
Potential Health Economic Value of Using Monocyte Distribution Width (MDW) in the Emergency Department (ED)

Shawn Schwartz
MBA,
Director, Market Access,
Beckman Coulter Diagnostics,
Brea, CA

Sarah Borah
MSBA, MT (ASCP),
Sr. Manager, Clinical Markets,
Beckman Coulter Diagnostics
BENEFACTOR SESSION
8:30 AM - 9:20 AM
hc1
Using Machine Learning and AI to Unlock the Full Value of Your Laboratory Data

Chuck Girard
VP, Data Strategy,
hc1,
Indianapolis, IN

Olivia Choudhury
PhD,
Senior Partner SA,
Healthcare & Life Sciences, AWS
BENEFACTOR SESSION
8:30 AM - 9:20 AM
ELLKAY
Utilizing Interoperability to Drive Efficiency and Value for Labs with Epic

Marci Dop
Vice President of Enterprise Lab Operations,
ELLKAY,
Elmwood Park, NJ

Joshua Bartosz
EHR Clinical Application Analyst II,
Jefferson Health,
Philadelphia, PA

Frank Beylo
Director of Operations & Technology, Laboratory Services,
Inova Health Systems,
Crofton, MD
MASTER CLASS
8:30 AM - 9:20 AM
“Best practices in Genetic Test Coding, Billing, Collections, with Prior-Authorization insights”
Molecular diagnostics leaders face persistent and unique challenges in growing their laboratory’s market share; several important dynamics can play crucial roles in overcoming these challenges, including accurate code assignment, understanding payor medical policies and prior authorization requirements, and maximizing efficient claims reimbursement with an effective appeals strategy. This session will provide a comprehensive overview of data-driven best practices in genetic test coding, billing, and collections, as well as insights into the prior-authorization process.
Proper coding is essential to a successful genetic program. With the ever-changing landscape of genetic testing, a failure to stay abreast of the latest coding guidelines and payor medical policies can significantly impact billing accuracy and timely reimbursement. Ms. Blattner will discuss common coding errors and how to avoid them, as well as strategies for effectively communicating with payors to resolve issues.
Ms Blattner will provide an overview of the billing process, including claims submission, what information is required, and how to follow up on unpaid claims. She will also outline strategies for optimizing revenue cycle management, and provide statistics demonstrating their impact to financial performance.
Prior-authorizations continue to be a prominent requirement in genetic testing, and Ms. Blattner will also cover strategies for developing a streamlined process for handling PAs that minimizes delays and rejections.
MASTER CLASS
8:30 AM - 9:20 AM
“Guerrilla Lean in Your Lab: Implementing Lean & Kaizen Events without Permission to Achieve Immediate Cost Savings, Improve Quality, and Boost Staff Productivity and Satisfaction”
In this presentation, the learner will understand the principles, tools and concepts of Lean applied to the healthcare environment. We discuss the “ground swell” or “boots on the ground Lean” that equip the worker with the tactics and tools to take control of their wasteful, inefficient, chaotic environment. The tactic called “Guerilla Lean” magnifies the impact of process improvement through development and empowerment of small groups within a local work-cell structure. Through kaizen, we discuss engaging workers in combat to stop firefighting and to take control of their own worn and defective work processes. The aim is to achieve immediate cost savings, workplace productivity and employee satisfaction.
MASTER CLASS
8:30 AM - 9:20 AM
“Re-engineering the Classic Histology Laboratory: Enabling the Remote Histotechnologist with New Tools that Improve Productivity, Automate Processes, and Protect Quality”
MASTER SESSION
8:30 AM - 9:20 AM
“Positioning Lab Services to Meet the Changing Needs of Healthcare, Physicians, Patients, and Payers: How Five Primary Trends Create Profitable Opportunities for Your Laboratory”
Editors and writers at The Dark Report frequently talk to sources within the clinical laboratory industry about important trends, best practices to run a lab, and strategies to stay financially stable. In looking back at 15 months of coverage from January 2022 through March 2023, five trends emerged that stretch across various areas of lab operations yet share a common theme: The trends all offer paths to greater profitability for clinical labs and pathology practices. Learn what these trends are, how real-life laboratory leaders took advantage of them, and why these approaches can work at other labs.
The trends we’ll explore include:
- Offering lab services in new, non-traditional care sites can provide additional sources of revenue.
- Hospital and medical labs may be missing a chance to derive full value from lab outreach programs.
- Thinking creatively about lab services opens the door to new revenue opportunities.
- Innovative laboratories are starting to put a dollar figure on the value of their diagnostic data.
- Establishing internal and external relationships can lead to budgetary advantages.

Scott Wallask
Editorial Director,
The Dark Report,
Spicewood, Texas

Jane Hermansen
Director, Outreach and Network Development,
Mayo Clinic,
Rochester, MN
MASTER SESSION
8:30 AM - 9:20 AM
Expanding IVD and Lab Vendor Market Share: Today’s Important New Differences How Lab Buyers Search Products, Select Vendors, and Make Buying Decisions

Ron Rohrer
Senior Sales Executive
The Dark Intelligence Group,
Indianapolis, IN

Deb Hewett-Smith
Principal,
Talking Labs!,
Spicewood, Texas
GENERAL SESSION
9:30 AM - 10:05 AM
“Rethinking the Lab’s Role to Support Precision Medicine, Serve the New Consumer, and Create Value from Data to Support Improved Patient Care and Save on Cost of Care”
GENERAL SESSION
10:05 AM - 10:45 AM
Driving Precision in Genetic Test Management Lab Benefit: Understanding the Value to Patients, Physicians, Lab Providers and Payers
In this interactive session leaders from Avalon and Optum will speak to precision medicine trends, the complexity of genetic test management and the need for innovative technology and genetic network solutions to ensure the right test is driving the right care with the right reimbursement.
Attendees will discuss:
- How prior authorization works for genetic test management and the role technology can play in automating PA where appropriate
- The need for evidence-based genetic test coverage guidelines- both clinical and payment implications
- How Z-codes will ensure test transparency, test quality and fast-tracking reimbursement that will benefit patients, providers, labs as well as health plans
- What health plans are looking for in a genetic test network and the value to Labs to participate

Cristi Radford
MS, CGC,
Product Director, Optum,
Eden Prairie, MN

Jason Bush
PhD, Executive Vice President,
Product, Avalon Healthcare Solutions, Tampa, FL
AM BREAK
10:45 AM - 11:00 AM
AM Break sponsored by Accumen
GNERAL SESSION
11:00 AM - 11:35 AM
“How We Used Agribusiness Technology to Create Ultra-High Throughput PCR Testing during the Pandemic: Lessons Learned, Current Use, and New Opportunities to Directly Serve Consumers Going Forward”
GENERAL SESSION
11:35 AM - 12:10 PM
Delivering More Value to Physicians, Patients, and Payers with Lab Testing Services: How Labs Can Best Meet Healthcare’s Changing Needs

Robert Michel
President,
The Dark Intelligence Group,
Austin, TX

Jason Bush
PhD, Executive Vice President,
Product, Avalon Healthcare Solutions, Tampa, FL

William Morice
MD, PhD,
CEO and President,
Mayo Clinic Laboratories,
Rochester, MN

Cristi Radford
MS, CGC,
Product Director, Optum,
Eden Prairie, MN
LUNCH
12:10 PM - 1:30 PM
Networking Lunch sponsored by Dark Daily
ROUNDTABLE
1:10 PM - 3:00 PM
“Academic Pathology Roundtable”
This workshop is an opportunity for members of academic departments of pathology and interested industry partners to share in their successes, identify challenges – recognized and not yet recognized, and learn about effective approaches to protecting and enhancing the role of academic pathology in these rapidly changing times. This roundtable is a highly interactive session, in which questions are teed up by the facilitators, and discussion given by all participants.

James Crawford
MD, PhD,
Senior Vice President of Laboratory Services, Northwell Health, Greenvale, NY

Joann Li
PA, MPH,
Department Administrator/CFO, Columbia University,
New York, NY
PANEL
1:30 PM - 2:20 PM
Genomic Testing: How Labs and Payers Can Work Together To Achieve Better Outcomes and Health Equity

Karen McFadden
Retired SVP,
LabCorp,
Chapel Hill, NC

James Almas
MD,
National Medical Director, LabCorp,
Burlington, NC
PANEL
1:30 PM - 2:20 PM
Clinical Laboratory Certification and Accreditation: New Developments, Most Common Deficiencies, and Making Your Lab “Assessment Ready”

Nora Hess
MBA, MT (ASCP), PMP,
Senior Consultant, Accumen

Denise Driscoll
MS, MT (ASCP) SBB,
Senior Director, Accreditation and Regulatory Affairs, College of American Pathologists

Kathy Nucifora
MPH, MT (ASCP), Chief Operating Officer, COLA Inc.

Amy Null
MBA, MT (ASCP), SBB,
Associate Director,
Standards Interpretation Group, Laboratory Accreditation,
The Joint Commission,
Oakbrook Terrace , IL,
PANEL
1:30 PM - 2:20 PM
Healthcare Data Interoperability: Opportunities for Labs and Updates on Federal Law, Regulation, and Enforcement

Sara Shanti
Partner,
Sheppard Mullin,
Chicago, IL

Alice Leiter
Attorney,
Manatt Health & Phillips,
Los Angeles, California
BREAKOUT SESSION
1:30 PM - 2:20 PM
“Advancing Digital Pathology Adoption with Effective Workflow and Informatics Changes from Histology and Scanning through Diagnosis, Reporting and Billing”
BREAKOUT SESSION
1:30 PM - 2:20 PM
“Community Hospital Success and Revenue Generation with Laboratory Outreach: What We Learned in Earning the Test Referrals from 100% of Our System’s Office-Based Physician Practices”

Sanjay Timbadia
MBA, BSc. MT (ASCP),
Director Of Laboratory Services,
Tucson Medical Center,
Tucson,AZ

Sandy Richman
MBA, C(ASCP),
Director, Healthcare Advisory Services, ARUP Laboratories,
Salt Lake City, UT
BREAKOUT SESSION
1:30 PM - 2:20 PM
Why an ‘Innovation Center’ Is a Force Multiplier for Your Lab: How to Create Productive Collaborations, Implement New Ideas, and Drive Diversified Revenue
This session will focus on the development of Sonora Quest Laboratories’ Innovation Center of Excellence, including complexities of navigating innovation within a joint venture, and determining the organizational synergies required to support ideation and operationalization. We’ll provide lessons learned while standing up this new business unit, as well as case studies and best practices to illustrate how integrating an Innovation Center can accelerate revenue and improve your value proposition.

Tom Leggett
Director of Business Development,
Sonora Quest Laboratories,Phoenix, AZ

Sky Soom
Innovation Analyst,
Sonora Quest Laboratories,
Phoenix, AZ
BREAKOUT SESSION
2:30 PM - 3:20 PM
“Obtaining Favorable Coverage Decisions for Genetic Tests and Genome Sequencing Services: Essential Steps with Payers and Understanding What’s Next in Genome-based Diagnostic Technologies”
BREAKOUT SESSION
2:30 PM - 3:20 PM
Starting on the Road to In-House Next-Gen Sequencing: Going Live on a Speedy Timeline to Support Clinical Care and Generate Additional Revenue

Cynthe Sims
PhD, HCLD(ABB),
VP of Clinical Diagnostics,
Blackhawk Genomics,
San Diego, CA

Chris Emery
Vice President Business Development – Molecular Dx & Clinical Laboratory, Halo Precision Diagnostics,
Aliso Viejo, CA

Lony Lim
Vice President Laboratory Operations,
Halo Precision Diagnostics,
Aliso Viejo, CA
BREAKOUT SESSION
2:30 PM - 3:20 PM
Communicating Critical Results: How Our Lab Leveraged Electronic Notification with Clinical Teams to Shorten Communication Times and Improve Patient Care

Deanna Franke
PhD, DABCC,
Technical Director – Core Laboratory,
Atrium Health,
Gastonia, North Carolina

Ryan Matos
Beaker and Reporting Consultant,
Honeydew Consulting,
Wilder, VT
BREAKOUT SESSION
2:30 PM - 3:20 PM
Winning Strategies for Attracting, Hiring, and Retaining Top-Performing Lab Managers during the ‘Great Resignation

Melissa Hurst
PhD,
Healthcare Practice Lead |
Vice President, Executive Search,
Slone Partners,
Columbia, SC

Bridget Burke
Esq.,
Chief Business Officer,
Slone Partners,
Bernardsville, NJ
PANEL
2:30 PM - 3:20 PM
Insights into the Growing Adoption of Digital Pathology: Making the ROI Case, Redesigning Histology and Pathologists’ Workflow, and What to Expect with AI-Powered DP Tools”

Cory Roberts
MD, MBA,
President, Sonic Healthcare USA Anatomic Pathology Division,
Austin, Texas

Andy Moye
PhD,
CEO, Paige
New York, NY

Lisa-Jean Clifford
COO & Chief Strategy Officer,
Gestalt Diagnostics,
Spokane, Wash

J. Mark Tuthill
MD,
Division Head, Pathology Informatics,
Henry Ford Health System,
Detroit, MI
GENERAL SESSION
3:30 PM - 3:45 PM
Extracting the Important Lessons from the Executive War College 2023
Additional Sessions - Schedule TBD
SESSION
Schedule TBD
Engaging and Convincing Management to Approve and Fund Implementation of Digital Pathology

Sumie Edwards
Digital Pathology Project Manager,
ARUP Laboratories,
Salt Lake City, UT
Thursday, April 27 (Top)
REGISTRATION
7:30 AM - 3:45 PM
Registration
BREAKFAST
7:30 AM - 8:30 AM
Continental Breakfast
WORKSHOP 1
8:00 AM - 4:00 PM
Genetic Testing: From Specimen to Reimbursement: Improving Patient Outcomes Every Step of the Way

Honey Reddi, PhD, FACMG
Professor & Chief, Division of Precision Medicine and Cytogenetics,
Medical College of Wisconsin

Diana Voorhees
MA, MLS, SH, CLCP, CPCO,
Principal/CEO
DV & Associates, Inc.
Salt Lake City, UT

Arezou Ghazani
M.Sc., Ph.D., FACMG,
Director of Clinical Genomics, Brigham Genomic Medicine,
Brigham and Women’s Hospital,
Boston, MA

Diana Richard
Sr. Director, Pathology and Strategic Development, XiFin,
San Diego, CA

Matthew Lebo
PhD,
Director of Bioinformatics,
Harvard Medical School,
Cambridge, MA

Matthew Stachowiak
PhD,
VP, Product Innovation,
GenomOncology,
Cleveland, Ohio

Laura Tafe
MD, Associate Professor of Pathology & Laboratory Medicine,
Dartmouth Hitchcock Medical Center,
Lebanon, NH

Bob Newton
MBA,
Healthcare Systems Executive,
Thermo Fisher Clinical Next Gen Sequencing Division,
Chicago, IL

Stan Cherel
Vice President, Sales,
Sophia Genetics,
Boston, Mass.

Thuy Phung
MD, PhD,
Medical Director,
University of South Alabama,
Mobile, AL

David Belfiore
Vice President, Sales,
Pillar Biosciences,
Natick, MA

Mehdi Keddache
Informatics Specialist,
Illumina Inc.,
Fort Lauderdale, FL

Sean Hofherr
PhD, FACMG,
Chief Operating Officer & Clinical Director, Fabric Genomics,
Oakland, CA

Samantha Lewis
PhD, SMB(ASCP) CM,
Clinical Development & Training Specialist,
Promega Corporation,
Madison, WI
More and more hospital labs and local labs are recognizing the opportunity to do genetic testing and whole genome sequencing on-site for several important benefits. First, local genetic testing/genome sequencing slashes the time to an- swer, giving the local lab competive advantage over national labs. Second, doing these assays locally creates the oppor- tunity to generate additional revenue. Three, this local testing creates more loyalty with referring physicians and that, in turn, helps the lab expand market share, generate greater test volume, and benefit from the resulting economies of scale in lab operations.
These are the reasons why you and your key team members will want to join us for this one-day intensive on establish- ing an on-site program of genetic testing and whole genome sequencing. You’ll learn what steps are required to accept a sample, extra and multiply the DNA, then generate the DNA sequences. Because information technology is essential to interpreting and reporting the resulting data, you will hear directly from experts the best and most cost-effective ways to accomplish this step and deliver accurate, timely results to referring physicians.
Also during this day of discovery, major suppliers for gene sequencing solutions, data interpretation solutions, and oth- er necessary services will be on hand, both as exhibitors and in presentations at our special product showcase. Add it all up and this is the perfect opportunity for you to get everything you need in one day to confidently kick-start your own lab’s on-site genetic testing and genome sequencing program. Insure your place by registering today!
AGENDA - WORKSHOP #1
| Wednesday | April 26, 2023 |
| 5:00 PM-7:00 PM | Reception and Exhibition |
| Thursday | April 27, 2023 |
| 7:00 AM-8:00 AM | Continental Breakfast and Exhibition |
| 8:00 AM-8:15 AM | Introduction and Overview Chair and Moderator: Honey Reddi, PhD, FACMG Professor & Chief, Division of Precision Medicine & Cytogenetics, Medical College of Wisconsin |
| 8:15 AM-9:30 AM | Pre-Analytical Considerations in Genetic Testing Laura Tafe, MD, Assistant Director of Clinical Genomics & Advanced Technologies, Dartmouth Health Vendor Solutions Presentations (10 minutes each): Illumina, Promega |
| 9:30 AM-10:00 AM | Morning Break and Exhibition |
| 10:00 AM-11:30 AM | Technologies Currently Used in Clinical Practice Arezou Ghazani, M.Sc., Ph.D., FACMG, Director of Clinical Genomics, Brigham & Women’s Hospital Vendor Solutions Presentations (10 minutes each): Illumina, Thermo Fisher, Biocartis, Pillar Biosciences |
| 11:30 AM-12:30 PM | Lunch and Exhibition |
| 12:30 PM-2:00 PM | Variant Analysis - Bioinformatic Pipelines and Reporting Considerations Matthew Lebo, PhD, Director of Bioinformatics, Harvard Medical School Vendor Solutions Presentations (10 minutes each): Illumina, Sophia Genetics, GenomOncology, Fabric Genomics |
| 2:00 PM-2:30 PM | Afternoon Break Reimbursement and Regulations for Genetic Testing |
| 2:30 PM-3:00 PM | A Brave New World or the Wild, Wild West? Understanding the Current Issues and Requirements to Successfully Bill and Collect for Genetic Tests and Genome Sequencing Services Diana Vorhees MA, MLS, SH, CLCP, CPCO, Principal/CEO, DV & Associates, Inc. |
| 3:00 PM-3:30 PM | Payment Opportunities in Genetic Testing and Whole Genome Sequencing: Understanding Payer Requirements and Proven Steps to Optimize Collections Diana Richard, Sr. Director, Pathology and Strategic Development, XiFin |
| 3:30 PM 3:45 PM | Guidelines for Genetic Testing and Sequencing: Laboratory Perspectives Honey Reddi, PhD, FACMG Professor & Chief, Division of Precision Medicine & Cytogenetics, Medical College of Wisconsin |
| 3:45 PM-4:00 PM | Summary and Concluding Remarks |
| 4:00 PM | END Genetic Testing/Genome Sequencing Workshop |
WORKSHOP 2
Thursday, April 27
8:00 AM - 4:00 PM
Lean Fundamentals for Lab Leaders: Proven Ways to Swiftly Slash Lab Costs While Boosting Quality and Improving Staff Retention

Charlie Protzman
CEO & Founding Partner,
Business Improvement Group

Dan Protzman
Partner and VP of
Customer Solutions,
Business Improvement Group
As you know, these are unprecedented times for the clinical laboratory profession. Lab costs are skyrocketing and supply chains are unreliable. Staff shortages are acute and lab managers are scrambling to manage overtime, minimize burnout, and retain top producers. These are all the reasons why you and your key managers need to join us for this special session on Lean Fundamentals.
Maybe you’re new to Lean. Or perhaps you’re looking for an even greater understanding of its principles and how to use them more effectively in your lab. Whatever your knowledge level, this special one-day Lean Boot Camp will give you all the information, skills, and confidence you need to take your lab to the next level.
Learn the Lean fundamentals and how to master many of its core concepts. Find out why Lean has emerged as one of the most powerful quality-management systems. Understand why the nation’s top-performing lab organizations use it to achieve the multiple benefits of smart cost-cutting and improving quality, even as it lowers stress levels, lifts morale, and significantly helps with staff retention across all functions and positions.
You’ll get techniques and best practices, all laid out in an easy-to-understand manner. Your leaders for this valuable session are Charles Protzman and Dan Protzman, both Master Black Belts with deep experience helping clinical labs and pathology groups successfully implement and sustain Lean.
This one-day intensive equips you with a solid grasp of Lean fundamentals, using exercises that demonstrate how they can transform your organization. Case studies and real-life examples will help bring these techniques to life. By the end of the day, you’ll be able to develop a continuous improvement roadmap for your own organization, with proven strategies for sustaining Lean in your lab. You will also receive a Certificate of Achievement for your Lean skills development and is recognized by your lab’s management team as a worthwhile accomplishment.
AGENDA - WORKSHOP #2
| 7:00 AM – 8:00 AM | Continental Breakfast and Exhibition |
| 8:00 AM – 8:30 AM | Introduction, expectation, ground rules, agenda, and what results to expect with Lean |
| 8:45 AM – 9:45 AM | Lab Workflow Game -Batch |
| 10:05 AM – 10:15 AM | Break and Exhibition |
| 10:15 AM – 10:55 AM | Batching vs. Lean (One Piece Flow) with video |
| 10:55 Am – 12:00 PM | Lean Business System Overview, Paradigm Video |
| 12:00 PM – 1:00 PM | Lunch and Exhibition |
| 1:00 PM – 1:30 PM | Lean and Change Management |
| 1:30 PM – 2:30 PM | Lean BASICS® Model and 6 Sigma Tools ▻ Video on Setup Reagent Change ▻ Video on Standard Work Improvement ▻ Video on Histology Embedding, Batch vs. single piece flow |
| 2:30 PM – 2:45 PM | Break |
| 2:30 PM – 3:45 PM | Lab Game Lean |
| 3:45 PM – 4:00 PM | Bringing it all together; process improvement exercise; Summary; Questions |
| 4:00 PM | End of Lean Fundamentals Workshop |
WORKSHOP 3
8:00 AM - 4:00 PM
‘Breaking Pathology’s Glass Slide’: Ways to Build Revenue with Digital Pathology, New Slide Scanning CPT Code, and More

Lisa-Jean Clifford
COO & Chief Strategy Officer,
Gestalt Diagnostics,
Spokane, WA

Dylan Miller
MD,
Pathology Department Chair,
Intermountain Health,
Salt Lake City, UT

Stephanie Fullerton
PhD,
Manager- Life science and Medical products, Hamamatsu Corp.,
San Leandro, CA

Jim Sweeney
President,
PathAI Diagnostics,
Memphis, TN

Ellen Beausang
MD,
Chief Commercial Officer / SVP,
BioReference Health,
Elmwood Park, N.J.

Jay McBurney
President & CEO,
LabRCM,
Houston, TX

Cory Roberts
MD, MBA,
President, Sonic Healthcare USA Anatomic Pathology Division,
Austin, Texas

Eric Walk
MD, PhD,
Chief Medical Officer,
Path.AI,
Boston, MA

J. Mark Tuthill
MD,
Division Head, Pathology Informatics,
Henry Ford Health System,
Detroit, MI

Sumie Edwards
Digital Pathology Project Manager,
ARUP Laboratories,
Salt Lake City, UT
AGENDA - WORKSHOP #3
| Thursday | April 27, 2023 |
| 7:00 AM – 8:00 AM | Continental Breakfast and Exhibition |
| 8:00 AM – 8:10 AM | Introduction and Overview Chair: Cory Roberts, MD (Sonic Healthcare USA) |
| 8:10 AM – 8:45 AM | Engaging and Convincing Management to Approve and Fund Implementation of Digital Pathology Sumie Edwards (ARUP Laboratories) |
| 8:45 AM – 9:20 AM | What Experience Teaches in Selecting the Best Digital Pathology Use Case for Your Lab Lisa-Jean Clifford (Gestalt Diagnostics) |
| 9:20 AM – 9:55 AM | Scanner and Viewer versus Fully-Integrated DP Platform: Why One is Limiting and the Other is Unlimited Dylan Miller, MD (Intermountain Health) |
| 9:55 Am – 10:25 AM | Break and Exhibition |
| 10:25 AM – 11:00 AM | Business, Medical and Technical perspective in selecting the preferred DP vendor, Implement the platform, and generate revenue from digital pathology - a panel discussion Ellen Beausang, MD (Bio-Reference Laboratories) |
| 11:00 AM – 11:35 AM | Understanding the Needs of Pharma, CROs, and BioResearch for De-Identified Samples and Data: Ways for Anatomic Pathology to Collaborate and Generate New Sources of Revenue Eric Walk, MD (Path.AI) |
| 11:35 AM – 12:35 PM | Lunch and Exhibition |
| 12:35 PM – 1:10 PM | How to Evaluate Scanners to Best Fit Various Digital Pathology Use Cases Stephanie Fullerton, PhD (Hammamatsu) |
| 1:10 PM – 1:45 PM | Achieving Interoperability with the DP System, Pathology LIS, and EHR: What's Easy, What's Not, and Secrets Learned the Hard Way Mark Tuthill, MD (Henry Ford Health) |
| 1:45 PM – 1:55 PM | Break |
| 1:55 PM - 2:30 PM | Experience in Using New Class III CPT Codes for Scanning and Creating Whole Slide Images Jim Sweeney (Path.AI-Poplar Health) |
| 2:30 PM - 3:05 PM | Current State for Anatomic Pathology Billing, Coding, and Collections: Payer's Responses to Advanced Diagnostic Services and Digital Pathology Jay McBurney (LabRCM) |
| 3:05 PM - 3:05 PM | Workshop Ends |
































