2025 Executive War College Agenda
Here is the 2025 Agenda, with announced session Titles and Speakers for the EWC Conference.
For registered attendees, this data will also be available on your Smartphone in the EWC Event App (Whova.) Access to this app will be provided soon.
We look forward to seeing you in New Orleans!
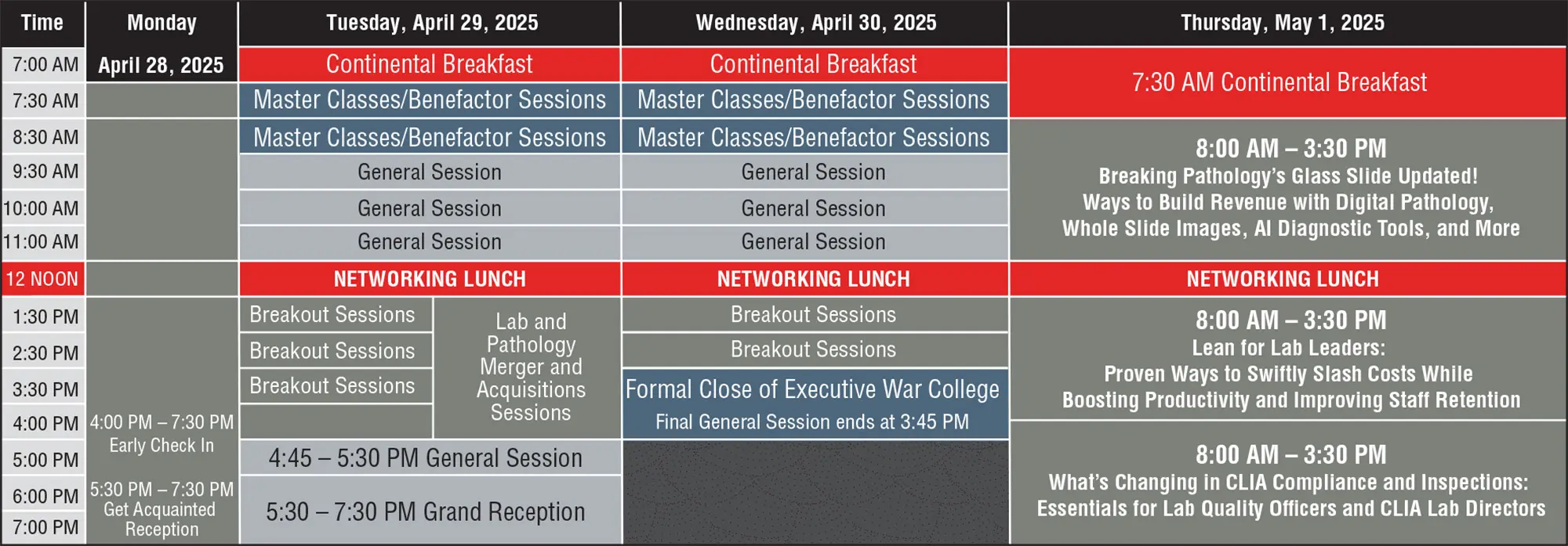
Monday, April 28 (Top)
REGISTRATION
4:00 PM - 7:30 PM
Early Check In
RECEPTION
5:30 PM - 7:30 PM
Get Acquainted Reception
Tuesday, April 29 (Top)
Don't Miss these Keynote Speakers!
Tuesday General Session — 9:30 AM - 12:10 PM
GENERAL SESSION
9:30 AM - 10:05 AM
Healthcare at a Tipping Point: Why Lab Opportunities and Challenges in Coming Years Will Be Different Than Those of the Past 30 Years
GENERAL SESSION
10:05 AM - 10:35 AM
Moving the Clinical Laboratory into the Age of Precision Medicine and All things Digital

Sam Terese
CEO & President,
Alverno Laboratories,
Gary, IN
AM BREAK
10:35 AM - 11:05 AM
BREAK
GENERAL SESSION
11:05 AM - 11:35 AM
Harnessing Innovation to Successfully Improve Lab Operations, Engage Vendors, Deliver More Value to Physicians and Patients

David Dexter
President/CEO,
Sonora Quest Laboratories,
Tempe, AZ
GENERAL SESSION
11:35 AM - 12:10 AM
Funding, Creating, and Operating the Nation’s first Diagnostic Center: Elevating the Value of Laboratory Medicine to Improve Patient Care in a Financially-Sustainable Model

Michael Laposata
MD, PhD, Professor and Chair, Department of Pathology,
Univ. of Texas Medical Branch,
Galveston, Texas
Tuesday Morning — Management Master Classes & Benefactor Sessions
MASTER CLASS
7:30 AM - 8:20 AM
New Revenue from Direct-to-Consumer Testing: Best Tests to Offer, Automating Processes, Engaging Physician Orders & Oversight, Tracking Consumer Payments, and Results

David Pellegore
Director of IT,
MaineHealth / NorDx,
Scarborough, ME

Patrick Schaffer
Chief Business Officer,
Lifepoint Informatics,
Glen Rock, NJ
MASTER CLASS
7:30 AM - 8:20 AM
Lab Testing Close to Where Patients Live and Work: How Our Lab Supports Urgent Care Clinics Tied to Freestanding Emergency Rooms at free standing EDS

Kathleen David
MT,
Associate Director,
Near Patient Testing,
TriCore Reference Laboratories,
Albuquerque, NM
MASTER CLASS
7:30 AM - 8:20 AM
Saving Staff Time and Money with Novel Solutions for Validation and Verification of Laboratory Instruments and Methods

Tim Bickley
VP, Sales US,
BYG4lab, Inc.,
Chicago, IL
MASTER CLASS
7:30 AM - 8:20 AM
Boosting Pathology Group Revenue with FNA to Offset Payer Cuts to 88305, Other CPT Codes and Protect Pathologist Compensation

Rahul Sukumar
CEO,
Doctors Pathology Services,
Dover, Delaware

Ray Sukumar
MD,
Doctors Pathology Services,
Dover, Delaware
MASTER CLASS
7:30 AM - 8:20 AM
Enabling Physicians to Order Genetic Testing Directly from the EHR: How it Works and Why It Benefits Patients and Labs

Mel Gamble
Director,
Digital Health,
Adaptive Biotech,
Seattle, Wash.
BENEFACTOR SESSIONS
7:30 AM - 8:20 AM
MedSpeed, WERFEN, Hamamatsu
BENEFACTOR SESSION
7:30 AM - 8:20 AM
MedSpeed
Fostering Innovation through Collaboration – A New Solution to Achieve Laboratories’ Operational and Financial Goals
Join us for an insightful session showcasing how leading labs are pioneering innovation with the support of Abbott and MedSpeed. This session will highlight how major labs are piloting a groundbreaking Indexor from Abbott in partnership with MedSpeed to transform the lab industry.
In this session, we’ll explore:
- An overview of Indexor and how it is helping labs meet their operational and financial objectives
- How MedSpeed and Abbott are collaborating to develop and pilot this technology
- Early results, lessons learned, and next steps for scaling the solution
Attendees will gain real-world insights into how organizations can leverage this strategic partnership to drive efficiency, enhance customer experience, and accelerate digital transformation. Don’t miss this opportunity to hear firsthand about the power of innovation through collaboration.

Andrew Roberts
Regional Vice President,
MedSpeed

Jake Sarosi
Digital Health – National Enterprise Accounts,
Abbott Diagnostics
BENEFACTOR SESSION
7:30 AM - 8:20 AM
WERFEN
Dedicated Hemostasis Automation: Optimizing Hemostasis Testing by Reducing Time to Result, Increasing User Efficiency, and Expanding Capacity Without Increasing Resources
This session will begin with an introductory speaker highlighting the macroeconomic forces that are compelling today’s labs to automate hemostasis testing. After establishing this premise, the speaker will emphasize the importance of automating hemostasis in the best way possible; specifically focusing on why automating hemostasis via total-laboratory automation (TLA) may not be ideal. These points will then be brought to life by Jayton Zachary, UMC Lubbock’s Clinical Laboratory Assistant Director, who will recount why he chose to implement dedicated hemostasis automation instead of TLA, how the technology has impacted his lab, and the significant performance improvements his lab has realized as it has worked to achieve maximum proficiency with dedicated hemostasis automation.

Jayton Zachary
Clinical Laboratory Assistant Director
UMC Health System,
Lubbock, TX
BENEFACTOR SESSION
7:30 AM - 8:20 AM
Hamamatsu
Unlocking the Data Behind the Glass to Empower the Agentic Future of Pathology
Digitization of pathology slides generates vast amounts of data—gigabytes per slide—representing an unprecedented opportunity for innovation. This session will explore how unlocking this immense volume of data can drive transformative workflows using advanced artificial intelligence (AI) tools.
Participants will examine state-of-the-art AI technologies, including specialized pathology applications and versatile multimodal systems, that leverage this digital data to significantly enhance diagnostic accuracy, efficiency, and patient care. We will introduce agentic workflows, AI-driven systems capable of autonomously managing complex tasks by integrating high-resolution pathology images, clinical records, genomics, and literature.
Through practical examples, attendees will discover how these data-driven, intelligent workflows can revolutionize diagnostics, research, education, and quality assurance. Join us to learn how effectively harnessing and analyzing large-scale digital pathology data can empower the pathology community, driving innovation and shaping the future of healthcare.

Matthew Cecchini
Pathologist,
London Health Sciences Centre and Western University
MASTER CLASS
8:30 AM - 9:20 AM
Serving Rural Patients Using Specimen Self-Collection: What’s Working with Micro-Collection and What’s Coming Next

Benjamin Bradley
Medical Director, Virology and Molecular Infectious Diseases,
ARUP Laboratories,
Salt Lake City, UT

Edwin Berthier
Co-founder and
Chief Technology Officer,
Tasso, Inc.
Seattle, Wash.

Leo Lin
MD, PhD,
Medical Director,
ARUP Laboratories,
Salt Lake City, UT
MASTER CLASS
8:30 AM - 9:20 AM
Challenges and Best Practices in Anatomic Pathology Charging: Connecting Beaker and EPIC EHR to Automate Data Collection to Improve Coding, Billing and Collections

Marshall Brett
Senior Lab Systems Analyst,
Wellstar Health System,
Atlanta, GA

Lily Sandler
Senior Beaker Consultant,
Honeydew Consulting,
Madison, WI

Zak Keir
Senior Beaker Consultant,
Honeydew Consulting,
Madison, WI
MASTER CLASS
8:30 AM - 9:20 AM
Automating Hemostasis Testing in Two Academic Center Labs To Achieve Faster TAT, Boost Staff Productivity, and Improve Auto-Validation Rates

Ryan Mize
DCLS, MHA,
Medical Director,
Massachusetts General Hospital,
Cambridge, MA.
MASTER CLASS
8:30 AM - 9:20 AM
New Digital Tools that Automate Processes to Reduce Billing Errors, Minimize Denials, and Accelerate Reimbursements: Case Studies and Examples from the Real World

Heather Agostinelli
VP,
Head of Specialty RCM,
XiFin, Inc.,
San Diego, CA

Atabek Yucel
Vice President,
Customer Technology,
XiFin, Inc.,
San Diego, CA
BENEFACTOR SESSIONS
8:30 AM - 9:20 AM
TELCOR, Sample Healthcare, LigoLab, U.S. HealthTek
BENEFACTOR SESSION
8:30 AM - 9:20 AM
TELCOR
Benefactor: How to Overcome Common Barriers to Reimbursement
Low collections are often attributed to two major challenges: flawed software that limits collections potential, and difficulties in finding skilled professionals or competent billing services. Addressing these barriers requires a strategic approach tailored to the unique needs of your laboratory.
For years, technology has lagged behind the growing complexities imposed by payers. Billing teams remain reliant on manual processes and outdated tools to manage prior authorizations, missing information, denials, and appeals. Deciding on the right software is critical but can be overwhelming. Should you opt for a cost-effective practice management system that may lack specialized functionality? Should you invest heavily in a robust solution with long-term ROI potential? Or should you consider an “out-of-the-box” solution that meets current needs but may lack scalability as your lab grows or regulations evolve?
Simultaneously, building a high-performing billing team has become increasingly difficult. The pandemic reshaped workforce dynamics and finding experienced revenue cycle professionals can feel like an uphill battle. While outsourcing billing operations to another country might seem like a quick fix, this approach often introduces new risks, including potential compliance challenges, data security concerns, and diminished control over operations. To succeed, leaders must adopt innovative approaches to recruitment—prioritizing skills, adaptability, and cultural fit over traditional criteria. This is equally crucial when vetting external billing services.
In this session, we’ll explore how to align your laboratory’s reimbursement strategy with the right technology and talent to maximize collections and streamline operations.
Key Takeaways:
- Strategically assess your lab’s billing needs to identify software solutions that meet operational, financial, and regulatory demands.
- Compare software options of practice management systems, enterprise solutions, and customizable platforms—to find the best fit for your organization’s maturity and growth trajectory.
- Reimagine workforce strategies by evaluating innovative recruitment practices and selecting billing partners who align with your lab’s goals.
- Understand the risks of offshore outsourcing and why keeping billing operations local ensures compliance, security, and greater operational control.

Darla Wanitschke
Vice President,
Customer Success,
TELCOR,
Lincoln, NE

Rick Roark
Consultant
BENEFACTOR SESSION
8:30 AM - 9:20 AM
Sample Healthcare
Snake Oil or Secret Weapon? Cutting Through the AI Hype in
Diagnostic Labs
In an era where “AI-powered” has become the most overused term in healthcare technology marketing, laboratory leaders face the challenge of separating genuine innovation from expensive disappointments. Sample Healthcare founders Ankit Ranjan and Aash Jain will expose the common pitfalls of AI implementations that fail to deliver while highlighting the specific applications actually transforming laboratory operations. Drawing from their research at University of Pennsylvania, Oxford, and MIT, along with their work with Adaptive Biotechnologies and Ambry Genetics, they’ll demonstrate how to identify AI solutions that address real laboratory pain points rather than creating new problems disguised as innovation. Attendees will learn why most AI deployments fail to achieve ROI and how to avoid making the same costly mistakes.
The session cuts through technical jargon to provide actionable evaluation frameworks based on real-world implementations. They’ll share case studies showing both successes (a 68% reduction in clinical review time) and failures (million-dollar implementations that never moved beyond pilot phase). Technical discussions will reveal how to distinguish between genuine advances in model architecture and cleverly repackaged conventional automation being sold at AI premium prices.
Attendees will gain insights into:
- How to spot “fake AI” through specific questions that expose marketing hype versus genuine technical capabilities
- Evaluation criteria for determining whether an AI solution can truly handle laboratory-specific challenges versus generic approaches that fail in specialized contexts
- Warning signs that indicate an AI implementation will likely fail to deliver promised benefits
- Practical applications where language models have demonstrated measurable laboratory ROI versus areas where the technology isn’t mature enough
- Implementation strategies that prevent pilot purgatory and ensure successful deployment
- A BS-detection framework for evaluating vendor claims about AI capabilities in laboratory settings

Ankit Ranjan
Founder,
Sample Healthcare

Aash Jain
Founder,
Sample Healthcare
BENEFACTOR SESSION
8:30 AM - 9:20 AM
LigoLab
From Bottlenecks to Breakthroughs: How Avero Diagnostics
Future-Proofed its Lab
In a competitive and rapidly evolving diagnostic landscape, Avero Diagnostics, a division of Versant Diagnostics recognized the urgent need to eliminate operational bottlenecks and position itself for long-term growth. By partnering with LigoLab, the lab made a strategic move that has transformed its workflows, boosted transparency, and enhanced financial performance.
Before the partnership, Avero faced challenges familiar to many independent labs: siloed systems, manual lab billing processes, limited visibility into revenue cycle performance, and frequent issues caused by disconnected LIS and RCM systems. These inefficiencies slowed down daily operations and limited the lab’s capacity to scale effectively.
The implementation of LigoLab’s fully integrated LIS and RCM platform marked a turning point. With seamless data flow between clinical and financial systems, Avero now benefits from real-time eligibility verification, automated claim submission, and powerful denial management tools all within a unified informatics ecosystem. This level of automation and integration has reduced labor hours, improved data accuracy, and eliminated the need for costly third-party workarounds.
Avero’s leadership team also gained access to actionable analytics that provide daily – not just
monthly – insights into key metrics like charges, deposits, and reimbursement trends. These insights empower decision-makers to act quickly, spot issues before they snowball, and continuously optimize performance.
Perhaps most importantly, the partnership gave Avero the autonomy to control its billing operations without sacrificing transparency or oversight. This combination of operational efficiency and financial insight has positioned the lab to sustain profitability in today’s market and grow with confidence in the years ahead.
Thanks to its collaboration with LigoLab, Avero Diagnostics has turned its operational challenges into strategic advantages – proving that with the right tools and partners, future-proofing a lab is not only possible but practical.

Ryan Fortna
Pathologist,
Avero Diagnostics, a division of Versant Diagnostics
BENEFACTOR SESSION
8:30 AM - 9:20 AM
U.S. HealthTek
Digital AI-Powered Labs: From Concept to Actionable Results
In today’s evolving healthcare landscape, laboratories face challenges like rising operational costs, labor shortages, and expertise gaps. While digital technology and AI have the potential to address these issues, many discussions on AI remain theoretical, leaving labs uncertain about implementation. This panel aims to bridge that gap.
Join Dr. Eric Glassy, a leader in digital pathology, and Lynn Brock, Chief Innovation Officer at SagisDx, as they explore how AI, digital pathology, and innovative technologies can streamline laboratory operations. Dr. Glassy’s experience in digital pathology, combined with Lynn Brock’s success in integrating AI-driven solutions, will provide invaluable insights into practical applications of cutting-edge technologies.
The discussion will cover how digital pathology and AI can help overcome challenges like rising expenses, workforce shortages, and the need for specialized expertise. They will share strategies for driving digital transformation in labs, focusing on improving workflows, reducing costs, and addressing workforce gaps. The panel will also highlight the underwhelming nature of previous AI discussions, emphasizing actionable steps for seamless AI integration into laboratory environments.
Attendees will leave with a clearer understanding of how to leverage technology and AI in their labs, turning theoretical ideas into tangible improvements and positioning teams for long-term success in an increasingly challenging landscape.

Eric Glassy
MD, FCAP,
Affiliated Pathologists Medical Group

Lynn Brock
CIO,
SagisDX
LUNCH
12:00 PM - 1:45 PM
Networking Lunch
WORKSHOP
1:30 PM - 4:35 PM
Laboratory & Pathology Merger & Acquisition Workshop
Chair: Cristal Contini, Chair of M&A Practice, McDonald Hopkins
1:30 PM – 2:15 PM
Panel—Business and Marketing Strategies for Laboratories in Changing Times
PANEL
Chair: Elizabeth Sullivan
Panelists: John Horton, Jeff Ellis, Julie Ramage

Elizabeth Sullivan
Chair,
Healthcare Practice Group,
McDonald Hopkins,
Cleveland, Ohio

John Horton
Vice President,
Sales & Marketing,
Versant Diagnostics

Jeff Ellis
Managing Director & Co-Founder,
Cross Tree Capital,
Tampa, FL

Julie Ramage
Principal and Founder,
Harbor Precision Genomics Consulting,
Gig Harbor, WA
2:15 PM – 3:00 PM
Panel—Behind the Curtain: Buyers Share What Drives Lab Valuations
PANEL
Chair: Emily Johnson
Panelists: Patrick Walsh, Jim Billington, John Erwin

Emily Johnson
Member,
McDonald Hopkins LLC,
Cleveland, Ohio

Patrick Walsh
Managing Director,
Ziegler,
Nashville, Tennesse

Jim Billington
CEO,
Versant Diagnostics,
Grapevine, Texas

John Erwin
Partner,
Kilpatrick Townsend & Stockton,
Raleigh, NC
3:00 PM – 3:45 PM
Panel—Current Market for Hospital Lab Outreach Transactions: Structuring the Deal and Management Service Arrangements
PANEL
Chair: Cristal Contini
Panelist: Ken Cerney, Rick Cooper, Brad Bostic

Cristal Contini
Chair,
Mergers & Acquisitions Practice,
McDonald Hopkins,
Cleveland, Ohio

Ken Cerney
Vice President – Commercial Operations, Health Systems,
Labcorp,
Burlington, NC

Rick Cooper
Owner,
GIG Consulting;
Affiliate Advanced Strategic Partners,
Cleveland, Ohio

Brad Bostic
Founder, Chairman & CEO,
hc1,
Indianapolis, Indiana
3:45 PM – 4:35 PM
Panel—Lab Valuation Trends: Factors That Drive High Values and Multiples in Acquisitions of Independent Labs, Genetic Labs, and Pathology Groups
PANEL
Chair: Chris Jahnle
Panelists: Christal Contini, Anil Asnani, Cory A. Roberts

Chris Jahnle
Managing Director,
Haverford Healthcare Advisors,
Paoli, PA

Cristal Contini
Chair,
Mergers & Acquisitions Practice,
McDonald Hopkins,
Cleveland, Ohio

Anil Asnani
Senior VP,
Strategy & Corporate Development,
Labcorp,
Burlington, NC

Cory A. Roberts
MD, MBA,
Chief Executive Officer,
Sonic Healthcare USA,
Austin, Texas
BREAKOUT SESSION
1:45 PM - 2:35 PM
Achieving “One Organization, One Application”: Using a Single, Front-End, Do-All IT Platform to Eliminate All Paper, Unlock Savings, while Boosting Productivity and Client Satisfaction

Sonny Varadan
Chief Information Officer,
Sonora Quest Laboratories
BREAKOUT SESSION
1:45 PM - 2:35 PM
FDA’s Legal Framework for AI: How it applies to Digital Pathology, Image Analysis Algorithms, Software, and Clinical Decision Support Systems

Gail Javitt
Director,
Hyman, Phelps & McNamara, P.C.,
Washington, DC

Lisa Baumhardt
Principal Medical Device Regulatory Expert,
Hyman, Phelps & McNamara,
Washington, DC
BREAKOUT SESSION
1:45 PM - 2:35 PM
Combining Lean and 100% Digital Pathology Locally and Nationally to Produce Faster, More Accurate Diagnoses with Increased Productivity of Staff and Pathologists

David Clark
MD,
Consultant Haematopathologist, Clinical Lead for Digital Pathology, Nottingham University Hospitals
NHS Trust,
Nottingham, England, UK
BREAKOUT SESSION
1:45 PM - 2:35 PM

Jennifer Fralick
Vice President, Anatomic Pathology & Clinical Laboratories
Stanford Health Care,
Standford, CA
BREAKOUT SESSION
1:45 PM - 2:35 PM
Bringing Phlebotomy Into the Digital Age: First Look at the World’s First Autonomous Blood Drawing System Now in Clinical Use

Brooke Katzman
Dr.,
Director of Hospital Clinical Laboratory, Point of Care, and Laboratory Services,
Mayo Clinic,

Thijs van Holten
PhD,
Clinical Chemist,
St. Antonius Hospital,
Nieuwegein, The Netherlands
BREAKOUT SESSION
1:45 PM - 2:35 PM
Diagnostic Center UTMB; Questions and Answers about Organizing and Operating a Diagnostic Center: Secrets, Successes, and Useful Insights

Michael Laposata
MD, PhD, Professor and Chair, Department of Pathology,
Univ. of Texas Medical Branch,
Galveston, Texas
BREAKOUT SESSION
1:45 PM - 2:35 PM
Harnessing New Digital Tools to Speedily Automate Existing Manual Work Processes in Support of Dynamic Hospital Laboratory Outreach Business Launch

Brice Bruno
System Vice President,
Pathology & Laboratory Services,
Wellstar Health System,
Lawrenceville, GA

Al Hirari
Co-Founder, Chief Revenue Officer,
1Health,
San Francisco, CA
BREAKOUT SESSION
1:45 PM - 2:35 PM
Eye on IVD Industry: New Trends in How Labs Buy Automation, Analyzers, IT, and AI
PANEL
Chair/Moderator: Bob McGonnagle
Panelists: Bruce Carlson, Lawrence Worden

Bob McGonnagle
Publisher, CAP TODAY,
College of American Pathologists,
Northfield, IL

Bruce Carlson
Publisher,
Eye On IVD LLC,
New York, NY

Lawrence Worden
Principal,
IVD LOGIX LLC,
Dallas, Texas

Rob LaCroix
Executive Director,
LTC LLC Healthcare,
Diagnostics & Life Science,
New York, NY
BREAKOUT SESSION
2:45 PM - 3:35 PM
Transforming the Patient’s Clinical Laboratory Experience: Novel Specimen Collection Solution Now Offered in Retail Pharmacies also Gives Labs a Unique Way to Add Value, Build Test Referrals

Eric Olson
Founder, Chief Operating Officer,
and Chairman
Babson Diagnostics,
Austin, TX
BREAKOUT SESSION
2:45 PM - 3:35 PM
Big Changes in Washington, DC: New Congress, New Administration, FDA, CMS, OIG, and More
PANEL
Moderator: John Kolozsvary
Panelists: Susan Van Meter, Russell Ring, Zach Rothstein, Erin Morton

John Kolozsvary
CEO,
JVHL,
Allen Park, MI

Susan Van Meter
President,
American Clinical Laboratory Association,
Washington, DC

Russell Ring
Vice President, Government Affairs,
Roche Diagnostics,
Washington, D.C.

Zach Rothstein
Executive Director of AdvaMedDx,
Washington, D.C.

Erin Morton
Partner,
CRD Associates,
Washington DC
BREAKOUT SESSION
2:45 PM - 3:35 PM
Winning with Hospital Lab Outreach: Today’s Proven Ways to Increase Test Volume, Improve Patient Outcomes, and Generate More Revenue

Jane Hermansen
Director,
Outreach and Network Development,
Mayo Clinic,
Rochester, MN
BREAKOUT SESSION
2:45 PM - 3:35 PM
Opportunities for Anatomic Pathology Labs to Collaborate with Pharma, Biotech, CROs: What Has Value for All Parties, Structuring Relationships, Identifying Productive Business Models
PANEL
Moderator: William Morice
Panelists: Jennifer Quigley, Jay White, Omar Perez, Robert Feeney

William Morice
MD, PhD
President and CEO,
Mayo Clinic Laboratories,
Rochester, Minnesota

Jennifer Quigley
Head of Precision Health & Partnerships,
BioNTech,
Mainz, Germany

Jay White
PhD, MPH
Sr. Director,
Precision Medicine & Diagnostics,
Lilly Oncology,
Indianapolis, IN

Omar Perez
MD,
Head of Medical Diagnostics,
US Medical Oncology,
AstraZeneca,
Washington, DC.
BREAKOUT SESSION
2:45 PM - 3:35 PM

Michelle Bell
HT/QIHC(ASCP)CM,
Applications and Service Manager,Milestone Medical,
Austin, TX

Elia Rosetta
CEO,
Milestone Medical Technologies, Kalamazoo, MI

Vanessa Visinoni
Vice President,
Milestone SRL,
Sorisole, Italy
BREAKOUT SESSION
2:45 PM - 3:35 PM
Transforming RCM with Artificial Intelligence: How Northwell Health Laboratories Accelerated Reimbursement, Reduced Billing Errors, and Minimized

Joe Accurso
Vice President,
Core Lab Revenue Cycle,
Northwell Health,
New Hyde Park, NY
Jeff Carmichael
Senior Vice President – Engineering, XiFin, Inc.,
San Diego, Calif.
BREAKOUT SESSION
2:45 PM - 3:35 PM
In a Red-Hot Market: How Smart Labs Recruit and Retain Young Pathologists

Jen Barna
MD,
Senior Executive Recruiter
(Pathology Division),
Santé Consulting,
St. Louis, MO
BREAKOUT SESSION
3:45 PM - 4:35 PM
Latest Developments with FDA Oversight of LDTs: Court Cases, How to Comply and How to Operationalize and Analyze the Financial Impact of Compliance Now!

Jane Pine Wood
Counsel,
McDonald Hopkins, LLC,
Cleveland, OH

Valerie Palmieri
CEO/Founder,
Momentum Consulting,
Monroe, CT

Sheila Walcoff
Founding Principal & CEO,
Goldbug Strategies, LLC,
Gaithersburg, MD
BREAKOUT SESSION
3:45 PM - 4:35 PM
Successful Hospital Laboratory Outreach Program Launches from a Standing Start and on a Budget by Exceeding Physicians’ Expectations and Delivering Clinical Value

Angela Vetch
MPH, DLM,
Director, Laboratory Services,
Kootenai Health,
Coeur d’Alene, ID
BREAKOUT SESSION
3:45 PM - 4:35 PM

Kyle Dunn
Founder & CEO,
Hyperdrive Bio,
Rancho Santa Margarita

Julie Ramage
Principal and Founder,
Harbor Precision Genomics Consulting,
Gig Harbor, WA
BREAKOUT SESSION
3:45 PM - 4:35 PM
Legal/Regulatory Panel; Important Developments in Laboratory Legal, Regulatory, and Compliance Requirements
PANEL
Chair/Moderator: Charles Dunham
Panelists: Danielle Sloane, Andrew J. Weissenberg, Caitlin Forsyth

Charles Dunham
Principal Shareholder,
Greenberg Traurig LLP,
Houston, TX

Danielle Sloane
Attorney,
Bass, Berry & Sims,
Nashville, Tenn.

Andrew J. Weissenberg
Counsel,
Buchanan Ingersoll & Rooney PC,
Washington, DC

Caitlin Forsyth
Attorney,
Davis Wright Tremaine,
Seattle, WA
BREAKOUT SESSION
3:45 PM - 4:35 PM
State of Market Adoption for Digital Pathology By Largest Lab Organizations in the United States

Lisa-Jean Clifford
COO & Chief Strategy Officer,
Gestalt,
Spokane, WA
BREAKOUT SESSION
3:45 PM - 4:35 PM
MolDx and Z-Codes: What’s New, What’s Changing and What’s Coming Next

Gabriel Bien-Willner
MD, PhD,
Program Medical Director, MolDX;
Chief Medical Officer,
Palmetto GBA,
Columbia, SC
BREAKOUT SESSION
3:45 PM - 4:35 PM
Healthcare’s Ongoing Transformation: What’s Changing for Labs and Ways to Address Pre-Analytical Issues in Support of Patient Consumerism, Improved Test Quality and Use of AI
PANEL
Chair & Moderator: Jake Crampton
Panelists: Sam Terese, Eric Carbonneau, Jennifer Fralick

Jake Crampton
CEO,
MedSpeed,
Elmhurst, Illinois

Sam Terese
CEO & President,
Alverno Laboratories,
Gary, IN

Eric Carbonneau
Vice President and Chief Operating Officer,
Tricore Reference Laboratories, Albuquerque, NM

Jennifer Fralick
Vice President, Anatomic Pathology & Clinical Laboratories
Stanford Health Care,
Stanford, CA
BREAKOUT SESSION
3:45 PM - 4:35 PM
Our Journey to Primary Diagnosis with a Fully-Digital Pathology Workflow while Partnering with Radiology

Ulysses Balis
MD, Associate Chief Medical Information Officer,
Director of Pathology Informatics and A. James French Professor,
University of Michigan,
Ann Arbor, MI

Mustafa Yousif
Assistant Professor,
Breast Pathology,
Informatics Dept. of Pathology, Michigan Medicine,
Ann Arbor, MI
GENERAL SESSION
4:45 PM - 5:30 PM
Diving Deep into Artificial Intelligence in Healthcare and Diagnostics: The Experts Opine
PANEL
Chair: William Morice
Panelists: Sonny Varadan, Thomas Seay, Ulysses Balis

William Morice
MD, PhD
President and CEO,
Mayo Clinic Laboratories,
Rochester, Minnesota

Sonny Varadan
Chief Information Officer,
Sonora Quest Laboratories

Thomas Seay
Executive Director, AI Catalyst,
The Health Management Academy,
Arlington, VA

Ulysses Balis
MD, Associate Chief Medical Information Officer,
Director of Pathology Informatics and A. James French Professor,
University of Michigan,
Ann Arbor, MI
Don't Miss these Keynote Speakers!
Wednesday General Session — 9:30 AM - 11:50 AM
GENERAL SESSION
9:30 AM - 10:00 AM
Making Patient Data Uniform, Portable, Accessible, and Searchable Across All Participating Healthcare Organizations: What’s Working and What’s Coming

Paul Wilder
Executive Director,
CommonWell Health Alliance,
New York, NY
GENERAL SESSION
10:00 AM - 10:30 AM
Artificial Intelligence in Healthcare: Understanding Current Capabilities, What’s Working Today, and What’s Coming Next (Thomas’s Title: Demystifying AI in Healthcare: Key Risks, Real-World Benefits, and Strategic Considerations)

Thomas Seay
Executive Director, AI Catalyst,
The Health Management Academy,
Arlington, VA
AM BREAK
10:30 AM - 10:50 AM
BREAK
GENERAL SESSION
10:50 AM - 11:20 AM
Whole Genome Sequencing: How What’s Happening Today Is a Guide to the Future of WGS in Health and Diagnostics

Richard Gibbs
PhD,
Professor and Director,
Baylor College of Medicine,
Houston, Texas
GENERAL SESSION
11:20 AM - 11:50 AM
Disruptive Change Ahead: What to Know about Healthcare Today and What’s Coming Next

Ted Schwab
Innovator,
Schwab Tremblay Solutions,
Los Angeles, CA
Wednesday Morning — Management Master Classes & Benefactor Sessions
MASTER CLASS
7:30 AM - 8:20 AM
PANEL: Leveraging Interoperability to Produce Fully-Documented Claims to Collect More Money Faster at Less Cost
PANEL
Moderator: Greg Stein
Panelists: Aaron Liston, Adrian Brown

Gregory Stein
Founder, CEO,
Shadowbox, Inc.,
Carlsbad, CA

Aaron Liston
Chief Executive Officer,
AIMA Business and
Medical Support, LLC,
Cary, NC

Adrian Brown
RCM Billing Manager,
Providers’ Choice Laboratories,
Houston, TX
MASTER CLASS
7:30 AM - 8:20 AM
Using a Unified View of Clinical, Genetic, and Anatomic Pathology Results to Add Value for Physicians, Case Managers, Payers, Pharma, and Other Stakeholders

Gregg Church
President,
4Medica Inc.,
Marina del Rey, CA
MASTER CLASS
7:30 AM - 8:20 AM
Effective Ways to Meet Today’s Lab Staff Recruiting and Retention Challenge: Educating High Schoolers, Leveraging Social Media, Career Paths in the Lab, plus Some Secrets

Mike Baron
Executive Director of Clinical Laboratory Operations,
Wisconsin Diagnostic Laboratories
Milwaukee, WI
MASTER CLASS
7:30 AM - 8:20 AM
Anatomic Pathology’s New Specialist Business Model: Aligning Services that Deliver Value to Referring Physicians, Payers, and Subspecialist Pathologists Seeking More Independence

Tiffani Milless
MD,
Pathologist and Founding Owner,
Goldfinch Laboratory,
Des Moines, Iowa

Jared Abbott
MD, PhD,
Pathologist and Founding Owner,
Goldfinch Laboratory,
Urbandale, IA
MASTER CLASS
7:30 AM - 8:20 AM
Technology Developments in Genomic Testing: From PCR to NGS

Arezou Ghazani
M.Sc, PhD, FACMG,
Brigham and Women’s Hospital/ Harvard Medical School,
Boston, MA

Nilesh Dharajiya
MD, Founder,
Healthbit,
San Diego, CA
BENEFACTOR SESSIONS
7:30 AM - 8:20 AM
Leica Biosystems, Roche Diagnostics
BENEFACTOR SESSION
7:30 AM - 8:20 AM
Leica Biosystems
Realized Benefits of Digital Pathology
Digital pathology is becoming the new standard of care, transforming the practice of pathology with many new laboratories adopting this technology. Leica Biosystems invites you to join our interactive session with industry experts as they share their insights on optimizing your digital pathology investment. Additionally, we’ll discuss building a practical business case for digital pathology, maximizing ROI benefits inside and outside the laboratory, and leveraging AI to boost productivity and efficiency.
Panel Topics:
- Building a Practical Business Case for Digital Pathology
- Maximizing ROI Benefits Inside and Outside the Laboratory
- Leveraging AI to Boost Productivity and Efficiency
- Optimizing Your Digital Pathology Investment
Moderator: Mark Frushone,
Panelists: Orly Ardon, Cory A. Roberts, Derek C. Welch

Mark Frushone
Global Director,
Digital Pathology,
Leica Biosystems

Orly Ardon
PhD, MBA, Director,
Digital Pathology Operations, Memorial Sloan Kettering Cancer Center

Cory A. Roberts
MD, MBA,
Chief Executive Officer,
Sonic Healthcare USA,
Austin, Texas

Derek C. Welch
MD,
President, Chief Medical Officer, PathGroup
BENEFACTOR SESSION
7:30 AM - 8:20 AM
Roche Diagnostics
Revolutionizing Diagnostics: Utilizing Algorithms and AI to Transform Modern Laboratory Practices
This session will describe innovative new algorithms in areas such as Sepsis that change clinical practice by allowing for earlier, more accurate, and more automated identification and management of disease. This talk will also describe details of these tools and the outcomes around their use.

Peter McCaffrey
MD, MS, FCAP,
Chief AI Officer,
University of Texas Medical Branch,
Galveston, TX

Matthew Prime
BSc, MBBS, PhD,
MRCS International Business Leader for CDS and Algorithms Roche Information Solutions,
RotKreuz, Germany
MASTER CLASS
8:30 AM - 9:20 AM
Taming the Lab Logistics and Courier Beast: New Approaches that Accelerate the Specimen Journey, Enhance Specimen Integrity, and Even Improve Home Specimen Collection
PANEL
Chair: Robin Hooker
Panelists: Ken Bahk, Erwin Berthier, Lloyd Gravois, Carrie Glas

Robin Hooker
SVP of Marketing &
Strategic Partnerships,
BioTouch,
Atlanta, Georgia

Ken Bahk
Chief Strategy Officer,
Neurogen Biomarking,
Chicago, ILL

Erwin Berthier
Co-founder and
Chief Technology Officer,
Tasso, Inc.
Seattle, Wash.

Lloyd Gravois
AVP,
Supply Chain Logistics,
Ochsner Health System,
New Orleans, LA

Carrie Glas
Senior Manager of Strategic Sourcing and Procurement,
College of American Pathologists
MASTER CLASS
8:30 AM - 9:20 AM
2025 Payer Denial Impact Report Sneak Peek: Unveiling Latest Trends and Recommended Practices for Optimizing Clinical Lab, Hospital Outreach, and Pathology Claim Denials and Appeals

Diana Richard
AVP, National Accounts, Path/Rad/Health Systems,
XiFin, Inc.
San Diego, CA

Stephanie Sessions
VP, RCM Operations,
XiFin, Inc.
San Diego, CA
MASTER CLASS
8:30 AM - 9:20 AM
Transforming Histology with 21st Century Solutions: What’s New in Automation, AI Workflow Management, and Even Telehistology

Eric Feinstein
President & CEO,
Clarapath,
Hawthorne, NY
MASTER CLASS
8:30 AM - 9:20 AM

Shashi Shetty
PhD, FACMG,
Director Cytogenetics and CLIA Laboratory Director, University Hospitals and Case Western Reserve University, Nation Prion Disease Pathology Surveillance Center
Cleveland, Ohio

Janice Heinichen
Territory Sales Manager,
Bioinformatics Molecular
Health-AMERICAS,
Integrated DNA Technologies

Scott Toman
Vice President,
Sales and Business Development,
Pillar Biosciences
BENEFACTOR SESSIONS
8:30 AM - 9:20 AM
Quadax, Synergen, ELLKAY, XiFin, Coronis
BENEFACTOR SESSION
8:30 AM - 9:20 AM
Quadax
Stronger Together: How Labs and a Strategic RCM Partner Achieve Financial Excellence
Success in lab billing isn’t just about the technology or service you choose; it’s about the partnership. Too often, labs view Revenue Cycle Management (RCM) as a vendor relationship rather than a strategic alliance that drives financial success. In this session, we’ll uncover why a true partnership between labs and RCM is essential for maximizing revenue and reducing costs. Join us to discover how labs that shift from a transactional vendor mindset to a collaborative RCM approach see measurable improvements in reimbursement and cost reduction. We’ll highlight real-world success stories and share critical benchmarking metrics to assess your lab’s financial health and transform revenue.
Key Takeaways:
- RCM as a Partnership – Shifting from a vendor mindset to a true RCM partnership fosters transparency, collaboration, long-term solutions, and stronger financial outcomes.
- The Foundation of Financial Stability – Preventing denials through proactive claim strategies and automation leads to faster reimbursements and drives cost savings.
- Turning Denials into Revenue – A structured denial management strategy, backed by analytics, can significantly improve reimbursement and optimize revenue recovery.
- Measuring & Maximizing Your Lab’s Financial Health – Tracking key reimbursement metrics and benchmarking against industry standards helps labs optimize their revenue cycle for long-term success.

Chad Miles
Director,
Reimbursement Management,
Quadax

Patti Grey
AVP RCS implementation & Application Support,
Quadax

Shawna Burdick
Senior Director,
RCM Optimization
BENEFACTOR SESSION
8:30 AM - 9:20 AM
SYNERGEN Health
Transforming Front-End RCM: Real-World Use Cases for Automation & AI
While much attention is given to denials and appeals, the front-end of the revenue cycle often holds untapped potential for accelerating reimbursement and reducing downstream issues. For laboratories dealing with high claim volumes and complex payer requirements, optimizing front-end workflows is not just beneficial, it’s essential.
In this session, we’ll explore how AI and automation are revolutionizing order intake, pre-billing validation, and billing processes to create a seamless, error-resistant revenue cycle from the start. Drawing from real-world case studies, we’ll highlight how labs are using intelligent automation to ensure data accuracy, reduce claim rejections, and speed up the time to payment.

Sunil Konda
Chief Product Officer,
SYNERGEN Health
BENEFACTOR SESSION
8:30 AM - 9:20 AM
ELLKAY
More Ready Than You Think: Launching a Successful Outreach Program
Launching an outreach program may seem daunting, but many health systems are more prepared than they realize. In this engaging panel discussion, Bryan Health, JTG Consulting Group, and ELLKAY share the story behind Bryan Health’s successful outreach program—from assessing market needs and choosing the right partners to operationalizing the program in 2020. Learn how ELLKAY’s expertise helped get the initiative off the ground and how JTG Consulting Group provided operational guidance to drive success. Whether you’re considering an outreach program or looking to optimize an existing one, gain valuable insights and a roadmap for the future of outreach in hospital labs.

Marci Dop
VP of Enterprise Lab Operations,
ELLKAY

Jamel Giuma
President & CEO,
JTG Consulting Group

David Kubik
Client Operations and Integration Manager, Laboratory,
Bryan Medical Center

Christina Nickel
Laboratory Director,
Clinical and Anatomic Laboratory,
Bryan Medical Center
BENEFACTOR SESSION
8:30 AM - 9:20 AM
XiFin, Inc.
Changing the Narrative on Prior Authorization: A Collaborative, Programmatic Approach Is Essential
Navigating the increasingly complex prior authorization (PA) process presents significant challenges, especially for advanced molecular and genetic diagnostic testing, specialty medical devices, and remote patient monitoring services. While point solutions may offer an efficient first step for routine testing, a more programmatic approach is essential for addressing the intricacies of PAs for complex procedures. This session will illustrate how integrating PA strategies into the broader revenue cycle management (RCM) process provides the visibility and coordination necessary to achieve meaningful results.
Attendees will gain insights into:
- The challenges patients and providers face due to aggressive and opaque PA policies and how to advocate for coverage, patient access, and responsible reimbursement.
- Data-driven perspectives on tracking PA success: Does higher PA success correlate to improved payment outcomes? How many claims are denied for unrelated reasons?
- The critical role of specialized expertise, such as genetic counselors, in improving claim accuracy and reimbursement outcomes. Learn how deploying niche skill sets at strategic workflow points reduces rework and drives financial success.
- The improved operational and financial performance achieved by one XiFin customer who has adopted a collaborate, programmatic approach to managing prior authorizations.
- Recommend best practices shared by XiFin’s new PA partner, careviso, for maximizing PA success and overcoming common obstacles.
Join us to examine how a collaborative, programmatic approach to prior authorization can transform your RCM processes, optimize reimbursement, and deliver better patient and provider outcomes

Heather Agostinelli
VP,
Head of Specialty RCM,
XiFin, Inc.

Ellen Beausang
President,
Chief Revenue Officer,
Lighthouse Lab Services

Perry Dimas
Co-founder and
Chief Business Officer,
careviso
BENEFACTOR SESSION
8:30 AM - 9:20 AM
Coronis health
They’re Coming For You
Real Life Meets Risk
Practice Management & RCM
Patients | Regulators | Payers | Employees
- HRSA Investigations continue to blossom
- Medicare Fraud & Overutilization — DOGE + HHS + CMS + OIG
- NSA & Patient Awareness: intensive & expanding
- Inurement à la mode – so many ways, every day
- Billing for wrong stuff – unimaginable
- Innocence or recklessness – knew or should have known
- Relying on the unreliable, mostly samples of one
- Missing the simple with EEs – OT, Exempt vs Non-Exempt, testing, billing
- Vendors treated poorly Competitors: just don’t like you

Michael Ferrie
MM/MBA,
President, Laboratory & Pathology,
Coronis Health,
Fenton, MO

Jane Pine Wood
Counsel,
McDonald Hopkins, LLC,
Cleveland, OH

Elizabeth Sullivan
Chair,
Healthcare Practice Group,
McDonald Hopkins,
Cleveland, Ohio

Emily Johnson
Member,
McDonald Hopkins LLC,
Cleveland, Ohio
LUNCH
12:00 PM - 1:30 PM
Networking Lunch
BREAKOUT SESSION
1:30 PM - 2:20 PM
Open Discussion: Artificial Intelligence in Healthcare, Bg Data, and Laboratory Medicine

Thomas Seay
Executive Director, AI Catalyst,
The Health Management Academy,
Arlington, VA
BREAKOUT SESSION
1:30 PM - 2:20 PM
Data Monetization: How to Stop Others from Using Your Lab Data. How to Sell Data. How to Avoid Giving Rights to Your Data to Others

Emily Johnson
Member,
McDonald Hopkins LLC,
Cleveland, Ohio

Elizabeth Sullivan
Chair,
Healthcare Practice Group,
McDonald Hopkins,
Cleveland, Ohio
BREAKOUT SESSION
1:30 PM - 2:20 PM
Open Discussion: CommonWell Health Alliance’s Role in Advancing Data Interoperability

Paul Wilder
Executive Director,
CommonWell Health Alliance,
New York, NY
BREAKOUT SESSION
1:30 PM - 2:20 PM
Global Look at Adoption of Artificial Intelligence in Anatomic Pathology: Improving Accuracy, Shortening Time to Answer, and Boosting Productivity

Joseph Mossel
Co-founder & CEO,
Ibex Medical Analytics,
Tel Aviv, Israel
BREAKOUT SESSION
1:30 PM - 2:20 PM
Using Simple, Off-the-Shelf Digital Tools to Speed Processing of Lab Claims, Reduce Manual Touches, Increase Clean Claims, and Generate More and Faster Reimbursement

Rick VanNess
Co-Founder, Wuscott LLC.,
Albuquerque, NM

Orus Guerra
CEO,
Southwest Labs,
Albuquerque, NM
BREAKOUT SESSION
1:30 PM - 2:20 PM
Effective Ways to Meet Today’s Lab Staff Recruiting and Retention Challenge: Educating High Schoolers, Leveraging Social Media, Career Paths in the Lab, plus Some Secrets

Mike Baron
Executive Director of Clinical Laboratory Operations,
Wisconsin Diagnostic Laboratories
Milwaukee, WI
BREAKOUT SESSION
1:30 PM - 2:20 PM
Moving Lab Data and Digital Pathology Images to the Cloud: Why It’s Happening, Who’s Competing for Your Business, What You Should Know When Buying This Service

Chandra Holback
Director Business Development,
Optum,
Prairie, MN

Lisa-Jean Clifford
COO & Chief Strategy Officer,
Gestalt,
Spokane, WA
BREAKOUT SESSION
1:30 PM - 2:20 PM
Generating New Revenue by Bridging Pharma Research Needs with Anatomic Pathology Group Resources

David West
CEO,
Proscia, Inc.,
Philadelphia, PA
BREAKOUT SESSION
1:30 PM - 2:20 PM
PART A—Data analysis for Genomic Testing: Tips and tricks
PART B—Status of the FDA oversight of LDT: An Association of Molecular Pathologists (AMP) perspective

Julie Hirschhorn
PhD, HCLD,
Molecular Microbiology System Director, Geisinger,
Danville, PA

Annie Scrimenti
Director,
Public Policy & Advocacy,
Association for Molecular Pathology,
Rockville, MD
BREAKOUT SESSION
2:20 PM - 3:10 PM
Clinical Laboratory Certification and Accreditation: New Developments with CLIA, Most Common Deficiencies, and Making Your Lab “Assessment Ready”
PANEL
Chair & Moderator: Nora Hess
Panelists: Kathy Nucifora, Denise Driscoll, Melany Williams, Chris Gunning

Nora Hess
MBA, MT (ASCP), PMP,
Senior Consultant,
Hc1,
Sarasota, FL

Kathy Nucifora
MPH, MLS (ASCP),
Chief Operating Officer,
COLA Inc.,
Columbia, MD

Denise Driscoll
MS, MT(ASCP)SBB,
Vice President,
Laboratory Accreditation Programs,
College of American Pathologists,
Northfield, IL

Melany Williams
MBA, MLS(ASCP),
Director, Laboratory Accreditation, Subject Matter Expert,
The Joint Commission,
Oakbrook Terrace, IL

Chris Gunning
Director of Operational Excellence,
A2LA,
Fredrick, MD.
BREAKOUT SESSION
2:20 PM - 3:10 PM
Achieving High Performance Workflow in the Core Lab by Tighter Integration of Automation, Analyzers, Data Tools, and Continuous Improvement

Jamel Giuma
President & CEO,
JTG Consulting Group,
Miami, FL

Alicia Simon
MBA, MT (ASCP),
Director, Central and Shared Services Laboratory Operations,
Intermountain Healthcare,
Canyons Region, UT
BREAKOUT SESSION
2:20 PM - 3:10 PM
Making the Case for Adopting Digital Pathology and Achieving a Healthy ROI: Update on Digital Scanning CPT Codes, Plus Structuring a Department Fund to Support AI and DP

Robert Tessier
Panelist,
Panel of National Pathology Leaders, Woodbridge, CT

Allegra E. Klein
MBA, Executive Director,
Panel of National Pathology Leaders, Woodbridge, CT
BREAKOUT SESSION
2:20 PM - 3:10 PM
Solutions to Histotechnologist Shortage and Histology Backlogs: Blending Recruitment with Short-Term and Long-Term Outsourcing

James Madory
DO,
Director of Pathology Informatics, Medical University of South Carolina, Charleston, SC

Tom Schofield
CEO,
Splice Histology, Inc.,
Worcester, MA
BREAKOUT SESSION
2:20 PM - 3:10 PM
Career Coaching: Powerful Ways It Can Propel the Management Effectiveness of You and Your Lab Team: Peggy McKee

Peggy McKee
Career Coach,
Career Confidential,
Corsicana, TX
BREAKOUT SESSION
2:20 PM - 3:10 PM
Survey of Current Image-Based AI Tools and Methods, Plus Their Applications Across All Types of AP/CP Imagery

Jerome Cheng
Clinical Associate Professor,
University of Michigan,
Ann Arbor, MI
BREAKOUT SESSION
2:20 PM - 3:10 PM
PART 3—Panel Discussion: Genomic Testing Considerations plus Panel of Experts Answer Your Questions
PANEL
Facilitator: Honey Reddi
Panelists: Julie Hirschorn, Annie Scrimenti, Arezou Ghazani, Shashi Shetty,

Honey Reddi
PhD, FACMG,
SVP and Medical Director,
Belay Diagnostics,
Chicago, IL

Julie Hirschorn
PhD, HCLD,
Molecular Microbiology System Director, Geisinger,
Danville, PA

Annie Scrimenti
Director,
Public Policy & Advocacy,
Association for Molecular Pathology,
Rockville, MD

Shashi Shetty
PhD, FACMG,
Director Cytogenetics and CLIA Laboratory Director, University Hospitals and Case Western Reserve University, Nation Prion Disease Pathology Surveillance Center
Cleveland, Ohio

Arezou Ghazani
M.Sc, PhD, FACMG,
Brigham and Women’s Hospital/ Harvard Medical School,
Boston, MA
BREAKOUT SESSION
2:20 PM - 3:10 PM
PART 4—Translating Genomic Testing Innovations to the Clinic: An Entrepreneur’s Perspective: Brian Coe

Brian Coe
Chief Executive Officer,
Belay Diagnostics, LLC,
Chicago, IL
GENERAL SESSION
3:20 PM - 3:40 PM
Extracting the Important Lessons from the Executive War College 2025
Post-Conference Workshops
To see session topics and speakers, select the underlined title for that workshop

