2024 EWC Sponsor: PathogenDx
PathogenDx MISSION:
TO DELIVER NEXT-GEN ARRAYS FOR
BETTER HEALTH AND SAFETY.
CLINICAL DIAGNOSTICS WITH A DIFFERENCE
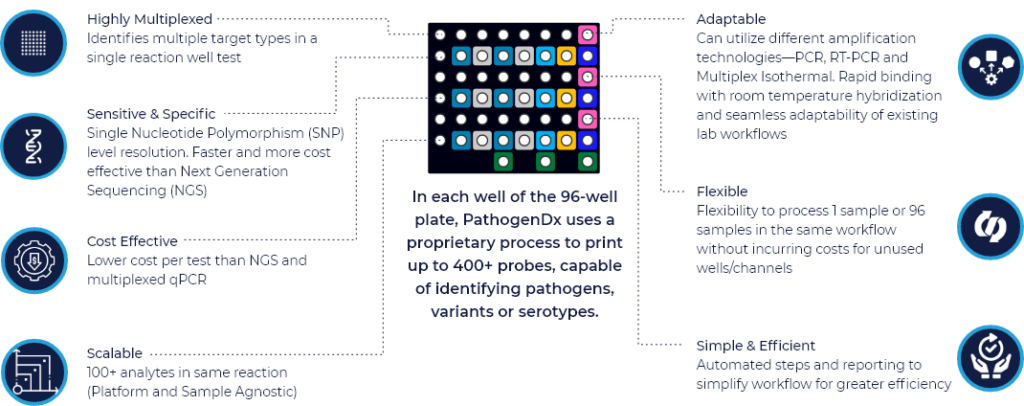
PathogenDx is harnessing the power of its D3 Array™ to provide RUO Assays that deliver more actionable information to reduce time in clinical decision-making. Discover how this new technology aids in selection and optimization of therapy and treatment plans.


UTI DETECTION & ANTIBIOTIC
RESISTANCE POWERED BY NOVEL
MULTIPLEXED MOLECULAR
DIAGNOSTICS TECHNOLOGY
D3 Array™-UTI is a fast, sensitive multiplexed PCR nucleic acid test that detects 26 pathogens and 12 antibiotic resistance markers—testing in triplicate, with qualitative and quantitative results in the same reaction AND automated, cloud-based analysis. Support clinicians in delivering rapid UTI treatment with truly disruptive diagnostics.
KEY FEATURES
Gain Ease – Test for 26 causative pathogens +12 Antibiotic Resistance Genes in a single test, with qualitative detection and quantification in the same sample reaction.
Cut Waste & Cost – Utilize only the wells you need per shift without incurring cost on the rest of the plate—optimizing reagents and consumables spend.
Streamline Analysis – Reduce the time and cost of deciphering reams of raw data via PDx’s Augury automated, cloud-based data analysis and management.
KEY BENEFITS

Identifies both the pathogen causing the infection and the antibiotic resistance gene marker in the same test. Affordable, accurate triplicate results in each reaction well.

Delivers Higher Accuracy with Triplicate Testing per target.
■ Quantitation is based on three measurements across three hypervariable regions
■ Built in positive and negative controls—no extra wells required

Equipment Agnostic for Greater Flexibility.
D3 Array™-UTI uses standard lab equipment to ease into your workflow and deliver ROI.

Gain results faster to keep your workflow humming.

Greater throughput vs other UTI panels—48 samples per plate.


