Combined cross-industry experience building, scaling, and digitizing clinical labs
CLIA/CAP/FDA/Specimen journey, bench process, instrument interfacing, and automation
Practice focus on LIMS, lab automation, dry and report related lab workflows
Practices focused on customer digital, lab master-data, EMR interfacing, preanalytical, logistics, client-service, genetic counseling, RCM, ERP, and more
“LEAP’s expertise in selecting the appropriate LIMS system for every clinical reference laboratory has enabled large-scale labs to achieve improved workflow efficiency, enhanced data management, and consolidated operations across departments and locations. By tailoring solutions to meet specific lab requirements, LEAP has helped laboratories accelerate revenue through faster time to market, streamline operations reducing cost per sample, always secure and always compliant.”
Discover a list of LEAP clients and high complexity genetics, oncology,
and m.o. Labs where LEAP’s team have all served.
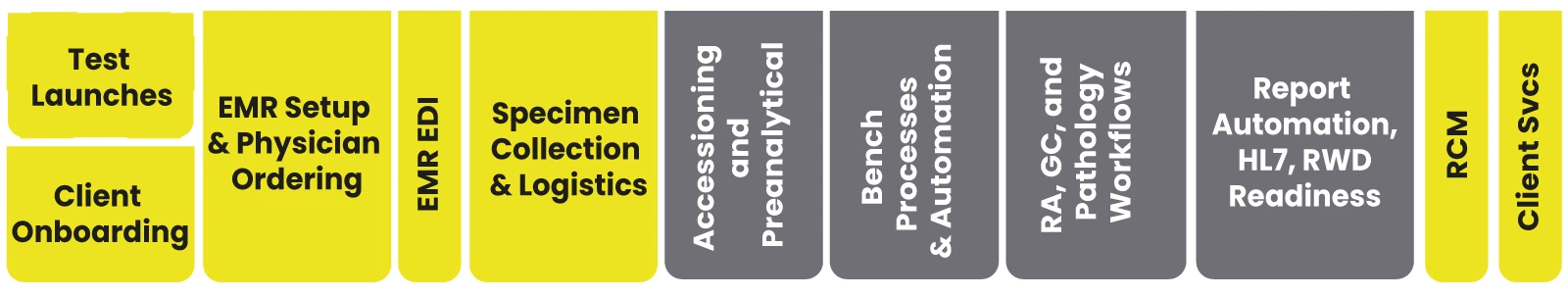
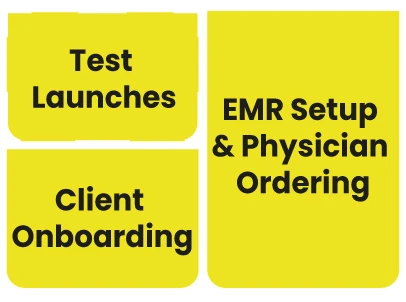
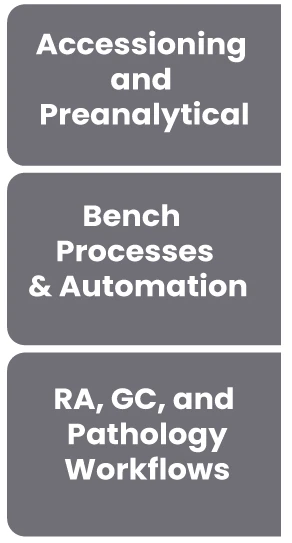
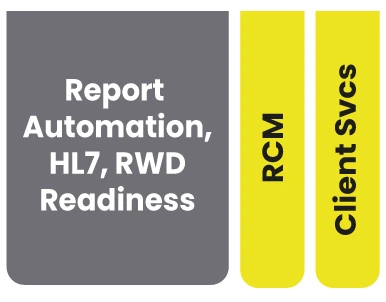
LEAP supports your lab from master data to provider experience to lab workflow to report writing, RCM, and everything else...

Business Model Variations (eg. [R-]TCPC)
Compendium & Medical Specialty Targeted Reqs (OncPath/GI/Uro/etc)
Exception Workflows - Adds/Deletes/TNPs
CLIA LDTs vs. FDA Lits | CAP testing vs. CFR 21
PART 11
RCM @ Ordering - Medical Necessity, Eligibility,
Prior Auth
Instruments & Technologies by Modality
by Lab Facility
Codified Results & Unstructured Result Artifacts
Sendouts, Reflexes, Stat/Rush
Patient Workflows - Results, Genetic
Counseling, etc
Specimen Tracking, Retention, and Return of Client Specimen
Enterprise-wide Data Assets - Operational Reporting, Quality Management, RWD Revenue-lines, Internal R&D and Publishing
Customer Lifecycle Reporting
Master Patient Indexing





LEAP is a full service consulting company advising and implementing across the technology lifecycle and ecosystem.
DIGITAL CXO ADVISORY SERVICES
Supply
-Functional Groups
-Leaders
-Skillsets & Teams
-Staff Aug & Vendors
-On Shore/Off-Shore
Demand
-Clients & Sponsors
-Sources of Demand
-Demand Planning
-Track Commitments
Connectivity
-Voice & Data Telco
-LAN/WAN/VAN
-Client & Vendor EDI
-Remote Work & CTI
Platforms
-Colo vs. Cloud
-IaaS vs. PaaS
-Custom vs. COTS
-Infra & Cloud Ops
Architectures
-Biz & Data Arch.
-Enterprise Arch.
-System Arch.
-Event & Data Maps
Info Sec & Comp
-Perimeter & Security
-Identities & Access
-Apps & Storage
-Audit & Docs
Goveranance
-Strategic Goals
-Roadmap Release
-Plans Mgmt
-Reporting
M & A
-Diligence
-Plan
-Integrate



